Reirradiation SABR
The Complexities of Dealing with Reirradiation Situations in SABR Treatment
Cancer treatment has advanced greatly. Patients are surviving longer with many now “living with” cancer rather than “dying of” cancer. Clinicians routinely combine multimodality therapies including surgery, chemotherapy, radiotherapy, immunotherapy, novel molecularly profiled targeted therapies, and local ablation techniques in a multidisciplinary fashion to offer truly personalised cancer medicine. This has extended survival significantly and improved quality of life for many cancers.
An increasing numbers of patients have received multiple courses of radiotherapy (SABR or conventionally fractionated) over the years, and the scenario of reirradiation has become more frequent. How do we deal with reirradiation situations? What recovery factors do we allow for organ tolerances? How long is “safe” between primary treatment and reirradiation? What dose should we give? What are the consent and governance risks around reirradiation treatment?
To many of these questions, there is no “correct” answer, and the published literature on reirradiation SABR consists of small heterogeneous retrospective studies; theoretical data on alpha/beta ratios and recovery factors is often based on historical animal experiments. Clinical trials in this arena are challenging due to the heterogeneous patient population. We must rely on clinical expertise, tight governance and close working with physics and dosimetry colleagues. This is truly individualised therapy, every reirradiation case has its own unique factors.
All cases of reirradiation SABR will be discussed by GenesisCare SABR Advisory Team (SAT); many patients are referred because they are not suitable for surgery or other local ablative techniques. Physics input is essential, and GenesisCare has developed a reirradiation SOP which includes a spreadsheet to calculate organ at risk (OAR) recovery factors based on the time since primary treatment and tissue alpha/beta ratio. Remaining organ tolerances can then be estimated, and we veer on the conservative side here. The most common sites for reirradiation include prostate, pelvic nodes, spine and liver. For reirradiation SABR it is crucial to minimise the volume of normal tissue reirradiated and thus there is often a benefit to treatment on the MR Linac. In the coming months GenesisCare will be developing further reirradiation protocols in conjunction with colleagues both in the UK and internationally.
As an illustration, a 64-year-old lady was first diagnosed with breast cancer in 2007, ER/PR negative and HER2 strongly positive. She underwent right mastectomy, FEC/Taxotere chemotherapy, radiotherapy to the right chest wall (40/15) and SCF and trastuzumab. Metastatic disease was first diagnosed in 2010, and over the years she was treated with a variety of systemic therapy agents including vinorelbine, Gemcitabine, Carboplatin, capecitabine and abraxane. Herceptin was continued throughout. In 2012 she underwent right hemihepatectomy for liver metastases, with a further liver resection for recurrent disease in 2013. In 2015 she received Cyberknife SABR to a hilar liver recurrence (field figure 1) 30Gy in 5 fractions – dose limited by adjacent colon, and in 2017 fractionated radiotherapy to the mediastinum for nodal recurrence (55Gy in 20 fractions). She continues to be chemo responsive to taxanes. In 2020 she developed a further 4cm liver metastasis in segment 2/4 (figure 2), for which resection was attempted but failed. She was then referred for further SABR treatment to the liver. There was partial overlap with the previous Cyberknife treatment field.
She was approved for reirradiation by SAT, and treatment on the MR Linac was recommended to minimise any overlap by judicious use of daily GTV and OAR recontouring and adaptation, daily plan reoptimisation and respiratory gated treatment delivery. With these measures we felt an ablative dose could be safely administered despite the previous SABR. The ability to account for interfraction and intrafraction motion on the MRL is especially important for reirradiation. The GTV was defined (figure 3) with a 2mm CTV and 3mm PTV margin and planned for a delivery of 50Gy in 5 fractions. Due to the conformality and steep dose gradient of MRL, 95% of the PTV received 50Gy, with a mean liver dose of just 8.2Gy (figure 4). Liver reirradiation SABR was delivered safely and with minimal toxicity (grade 1 fatigue and nausea). 50% recovery of liver and bowel tolerance was allowed from the previous SABR 5 years ago.
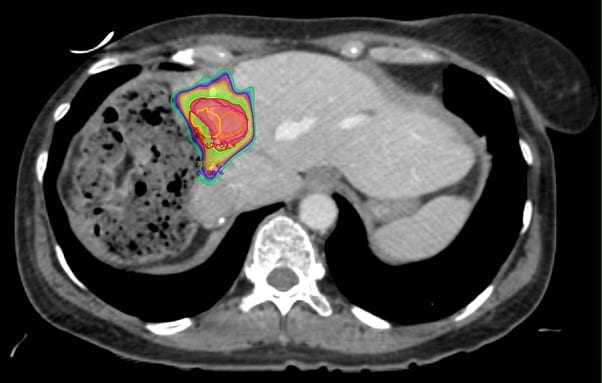
Figure 1 – Previous Cyberknife Treatment 2015, 30Gy in 5 fractions due to proximity of large bowel.
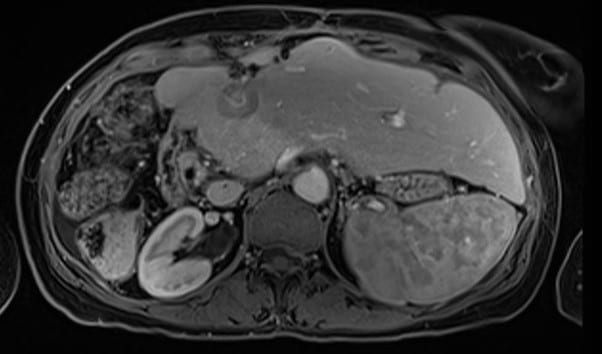
Figure 2 – Diagnostic MRI of “edge recurrence” liver metastasis 2020
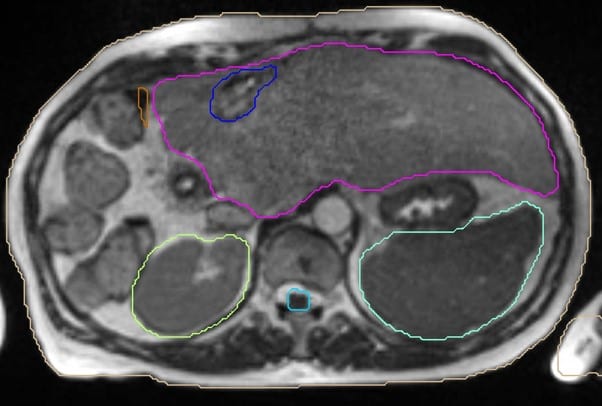
Figure 3 – GTV delineation (blue) for reirradiation volume.
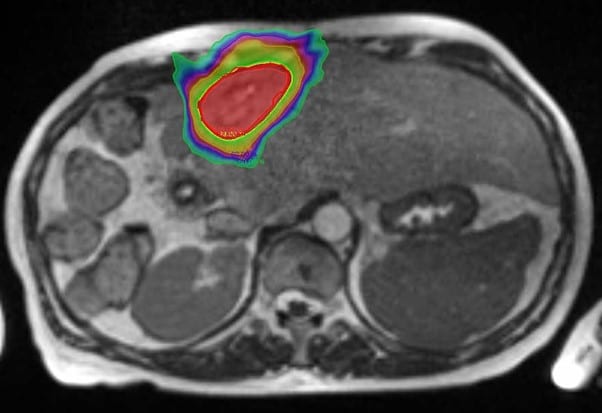
Figure 4 – MR Linac Reirradiation treatment plan – 50Gy in 5 fractions.
In summary, reirradiation SABR is feasible and safe but requires a careful multidisciplinary approach. GenesisCare has the expertise, governance framework and technology to enable effective reirradiation treatment. GensisCare’s SABR Advisory Team working in conjunction with physics and dosimetry ensure the physics and radiobiological aspects of retreatment are accounted for. The MR Linac, in particular, is extremely useful for reirradiation SABR as the volume of normal tissue reirradiated can be kept to the minimum with MRL’s daily adaptation and reoptimisation features.
Any questions please contact me directly or via sabr@genesiscare.co.uk
Kind regards,
Dr Andy Gaya
Clinical Oncologist and MRIdian specialist
GenesisCare