- Healthcare Professionals
- Resources
- Case studies
- MRI fusion vector prostate biopsy case study
Case study
MRI fusion vector prostate biopsy under local anaesthetic
Mr Christof Kastner

Mr Christof Kastner is a Consultant Urologist based in Cambridge and former Professor of Urology in Rome. He’s an internationally leading expert in assessing and treating benign and cancerous prostatic disease.
Having formed collaborations with colleagues around the world, his publications have contributed to the change in the approach to prostate cancer assessment.
In 2012 Mr Kastner and his team in Cambridge were the first to offer Transperineal Prostate (TP) Biopsies using MRI-US fusion guidance in the UK.
Case presentation
A 68-year-old male with suspected prostate cancer contacted GenesisCare for a second opinion. A rising PSA (from 5 to 7 ng/ml) during an annual health check prompted his GP to refer him for a prostate cancer assessment. He was otherwise fit, active and well. A prostate MRI was reported as normal at his local hospital.
With a wider range of diagnostic techniques available, he requested a further opinion by the team in Cambridge and GenesisCare to discuss his options.
Challenges of presentation and choice of treatment
We were able to offer the patient a second reading of his MRI which revealed a borderline significant lesion (Likert 3-4) on the edge of the outer zone of the prostate with a potential of touching the capsule (possibly T3a). Two options for biopsy were discussed: MRI fusion template-guided TP prostate biopsy under general anaesthetic or MRI fusion vector-guided TP prostate biopsies under local anaesthetic. The patient opted for MRI fusion vector TP biopsy due to the high targeting accuracy, favourable side effect profile and comfort of having the procedure under local rather than general anaesthetic.
Treatment
The patient’s MRI scan was re-reported by Professor Barrett, a Cambridge based GenesisCare expert prostate radiologist. It was concluded that there was a Likert 3-4 lesion in the right mid gland peripheral zone (PZ) (1).
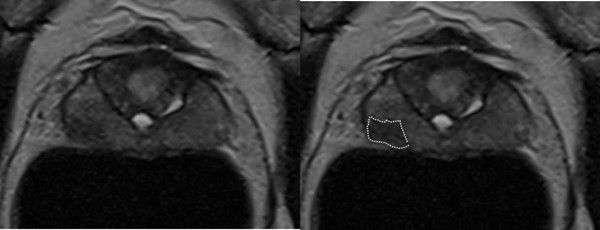
Figure 1: Contoured MRI
The patient then had an MRI fusion vector-guided prostate biopsy (2) targeting the suspicious lesion as well as across the prostate under local anaesthetic at GenesisCare in Cambridge. This was the first centre in the UK to offer this pioneering technique and remains a world leader in the field. The aim was either to accurately diagnose prostate cancer or to exclude it with certainty.
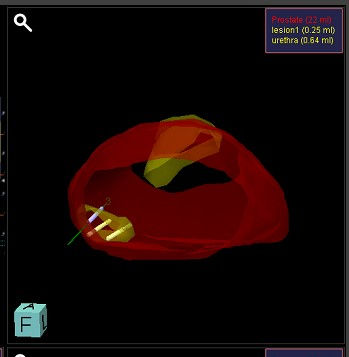
Figure 2: Targeted biopsy
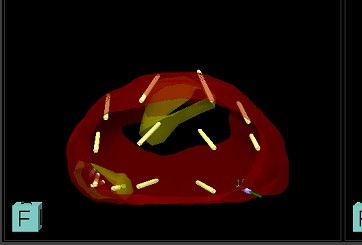
Figure 3: Systematic biopsy
During TP vector prostate biopsy, both targeted and systemic biopsies (3) can be taken. A stepper-mounted ultrasound probe is placed in the rectum and local anaesthetic is injected into the perineum. Using fusion software, the ultrasound image set is combined with the MRI (4).
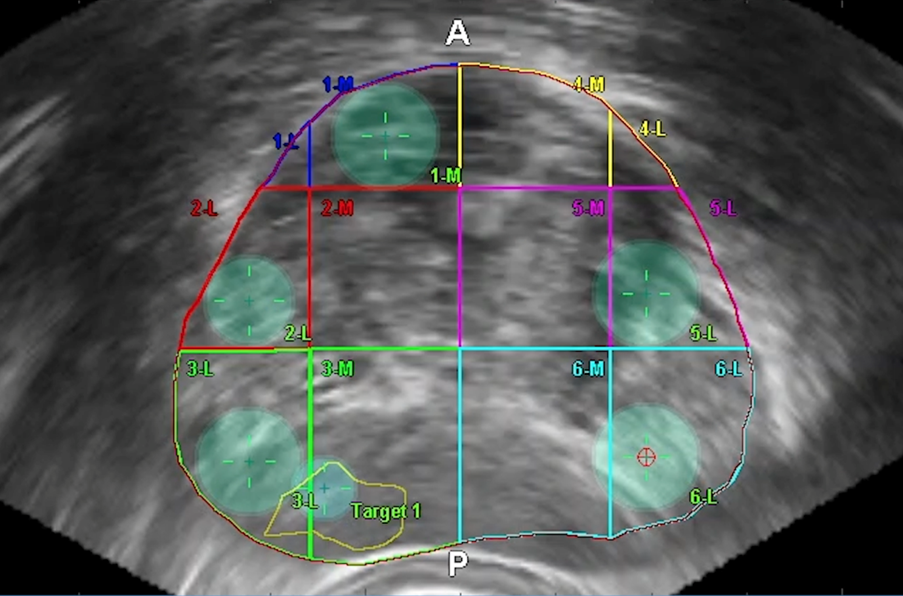
Figure 4: Crosshairs in prostate target
A needle sheath with an electromagnetic sensor is inserted into the perineum and the trajectory of the needle is tracked in an electromagnetic field. A circle shows when the needle trajectory cuts the ultrasound plane on the screen, allowing direction of the needle onto the lesion of interest for biopsy. The ultrasound probe remains stable and only two injections are needed in the perineum for samples, improving comfort for the patient.
With the high accuracy of TP vector prostate biopsy, the procedure can be carried out with minimal disruption for the patient. In this case, the length of time on the couch was around 20 minutes in total.
Side effects and management
No side effects were recorded, and the patient had no infection nor retention of urine. For further confirmation, urine dipstick tests at one and two weeks after the biopsies were negative.
As well as oncological outcomes, we have recorded patient-reported outcome measures (PROMs) for TP vector prostate biopsy since its introduction at GenesisCare. The patient reported that pain during the procedure was not an issue for him. He had some blood in the urine and stools for one day only. There was no effect on urinary or sexual function reported.
Results and follow-up
Of all biopsies taken the targeted biopsies of the suspicious lesion revealed the highest cancer grade and length confirming the precision of the technique. Prostate cancer was found with a Gleason score 4 + 3 = 7 (Grade group 3), in 5/15 cores with the longest tumour core being 11mm and intraductal carcinoma present.
With certainty of diagnosis, the team of urologists and oncologists at GenesisCare were able to present the patient with treatment options appropriate for his condition, which included robotic prostatectomy and radiotherapy. He opted for the latter and was started on hormonal treatment to prepare the prostate cancer cells prior to radiotherapy.
Discussion
Procedures for diagnosis of prostate cancer have evolved over time as knowledge, technology and experience have developed. Accurate diagnosis is crucial for determining appropriate treatment, whilst minimising unnecessary administration of treatment unlikely to offer clinical benefit. It is recommended that TRUS biopsies, which is associated with a risk of side effects and has limitations in diagnostic capabilities, be replaced by TP biopsies as standard of care. TP vector prostate biopsy represents the latest innovation in diagnostic techniques. This novel method using electro-magnetic needle tracking has consistently shown extremely high precision of targeting in comparison with other techniques. With just two perineal entry points, it has a favourable side-effect profile and reduced risk of infection or sepsis. As it can be carried out under local anaesthetic, convenience for the patient is improved. Research into TP vector prostate biopsy continues. Once fully evaluated, it is expected that this technique will permit a lower biopsy core number and therefore further reduced impact on patients.
In this case, the value of TP vector prostate biopsy for the patient was clearly demonstrated. His initial MRI reading had been unable to provide any diagnostic certainty. Understandably, the patient was seeking clarification so that he could either start appropriate treatment or have peace of mind that none was necessary. With superior accuracy to other diagnostic methods, the TP vector biopsy was able to provide clarity of his condition with minimal inconvenience. Although not the result any patient would wish for, it enabled prompt commencement of treatment with an improved prognosis compared to delay. Regarding the procedure, the patient reported that he was pleased with the process, team, and the procedure itself.
Get in touch
Contact us today to find out more about our vector prostate biopsies at our Cambridge and Windsor UrologyHub and how to refer a patient.
