- Healthcare Professionals
- Resources
- Case studies
- MRIdian case studies
Prostate – 9 months post treatment
Dr Philip Camilleri

MRI-guided ultra hypofractionated radiotherapy to the prostate – 9 months later
Case presentation
The patient, a 77-year-old male with prostate cancer, presented with a PSA level 6.8, stage T2cN0M0 with a Gleason score 3+4 = 7 (grade group 2). This gentleman was the first UK patient of GenesisCare to receive treatment on the MRIdian.
Challenges of presentation and choice of treatment
The patient was diagnosed in June 2019 with a multi-parametric MRI scan followed by transrectal ultrasound-guided biopsies. At the time it was felt that stereotactic ablative radiotherapy (SABR) would be an appropriate treatment. His treating uro-oncologist, Dr Philip Camilleri, was one of a group of consultants at GenesisCare undergoing training on MRI-guided SABR radiotherapy in preparation for the installation of the UK’s first MRIdian in October. This involved a close working relationship with VU University Medical Center (VUMC) in Amsterdam who already had two identical machines.
It was agreed that the patient would travel to Amsterdam as a guest of GenesisCare for treatment led by the team at VUMC.
MRIdian treatment
He was prescribed 36.25 Gy in five fractions. The treatment was undertaken in September 2019 and completed over alternate days with each session taking approximately 45 minutes to one hour to complete.
At each session, the target was recontoured and the daily plan adapted to allow for daily movement and bowel filling. The importance of live MRI-guided treatment was highlighted on day one when treatment had to be discontinued part way through due to excess wind, making it impossible to safely avoid organ at risk (OARs). The additional part treatment was completed on an additional day.
Results and follow-up
The patient experienced some mild side effects within the first four weeks after radiotherapy but no significant radiation-induced side effects have been recorded.
No patient-reported outcome measures (PROMs) data are recorded for this patient as it preceded the set-up of the UK service in Oxford.
In October, four weeks after treatment, the patient’s PSA level had reduced to 4.6. At the end of January, the PSA was 2.3 and in the first week of July 2020 his PSA level was 1.3.
The patient is currently in remission.
Discussion
This was the first UK patient to undertake this ultra hypofractionated treatment. Although he had to travel to Amsterdam, his treatment was completed in five sessions and he found it much easier to complete than expected, with limited toxicities leaving him feeling much better overall. Nine months after treatment, his PSA level continues to reduce as a positive sign of treatment efficacy.
Prostate – reirradiation
Dr Prantik Das

MR-guided re-irradiation for a prostate cancer patient unable to tolerate hormonal treatment
Case presentation
A 78-year-old male presented with a T3bN0 adenocarcinoma of the prostate, Gleason score 4+5.
The patient was treated in October 2018 with radical radiotherapy receiving 74 Gy in 37 fractions. He had a good biochemical response, with a PSA nadir of 0.08. In 2021, his PSA started to rise and reached 2.73. A PSMA PET scan showed PSMA avid local recurrence in the prostate only. He started on LHRH agonist but was experiencing extreme lethargy and hot flushes which had a significant impact on his QOL and difficulty in tolerating treatment.
He was referred to Dr Prantik Das to discuss the option of re-irradiation to his prostate. The patient had a previous history of thrombotic disease and bowel surgery but otherwise presented with a fit performance status of 0.
He was offered the option of prostate re-irradiation using MR guided stereotactic radiotherapy (SABR) at GenesisCare in Oxford. The option to deliver this treatment on the MR-guided system offers additional benefits. The daily adaption can allow for intra- and inter- fractional movement of the prostate and organs at risk (OARs), improving treatment accuracy and therefore reducing toxicity to healthy tissue. This could help to achieve a better quality of life (QoL) for this patient. It would also allow him to discontinue long-term androgen deprivation treatment androgen deprivation therapy (ADT) which adversely affected his QoL.
Preparation and treatment
A prostate biopsy confirmed his histologic recurrence. Furthermore, he received a rectal spacer insertion to reduce the rectal radiation dose and reduce gastrointestinal toxicity. The treatment dose of SBRT 30 Gy in 5 fractions was delivered on the MRIdian MR linac on alternate days. His treatment finished in February 2022 and subsequently the ADT was stopped after completing radiotherapy.
Results and follow-up
Early grade 2 bowel and grade 2 urinary toxicities were experienced, which settled within a few weeks.
At 3 months post-follow up, the patient described no bowel issues. His urinary side effects were back to his pre-treatment baseline (nocturia 2/3 times), and because he was able to stop his ADT the side-effects of tiredness had reduced and he was feeling significantly better within a few weeks.
At 9 months follow-up, the patient’s PSA value was 0.07.
Advantages of reirradiation
The MR guided reirradiation enabled the patient to undergo a potential curative treatment for his recurrent disease. This was achieved with moderate short-term toxicities and in doing so, it was possible to reduce the side-effects of long-term hormone treatments, greatly impacting the patient’s quality of life.
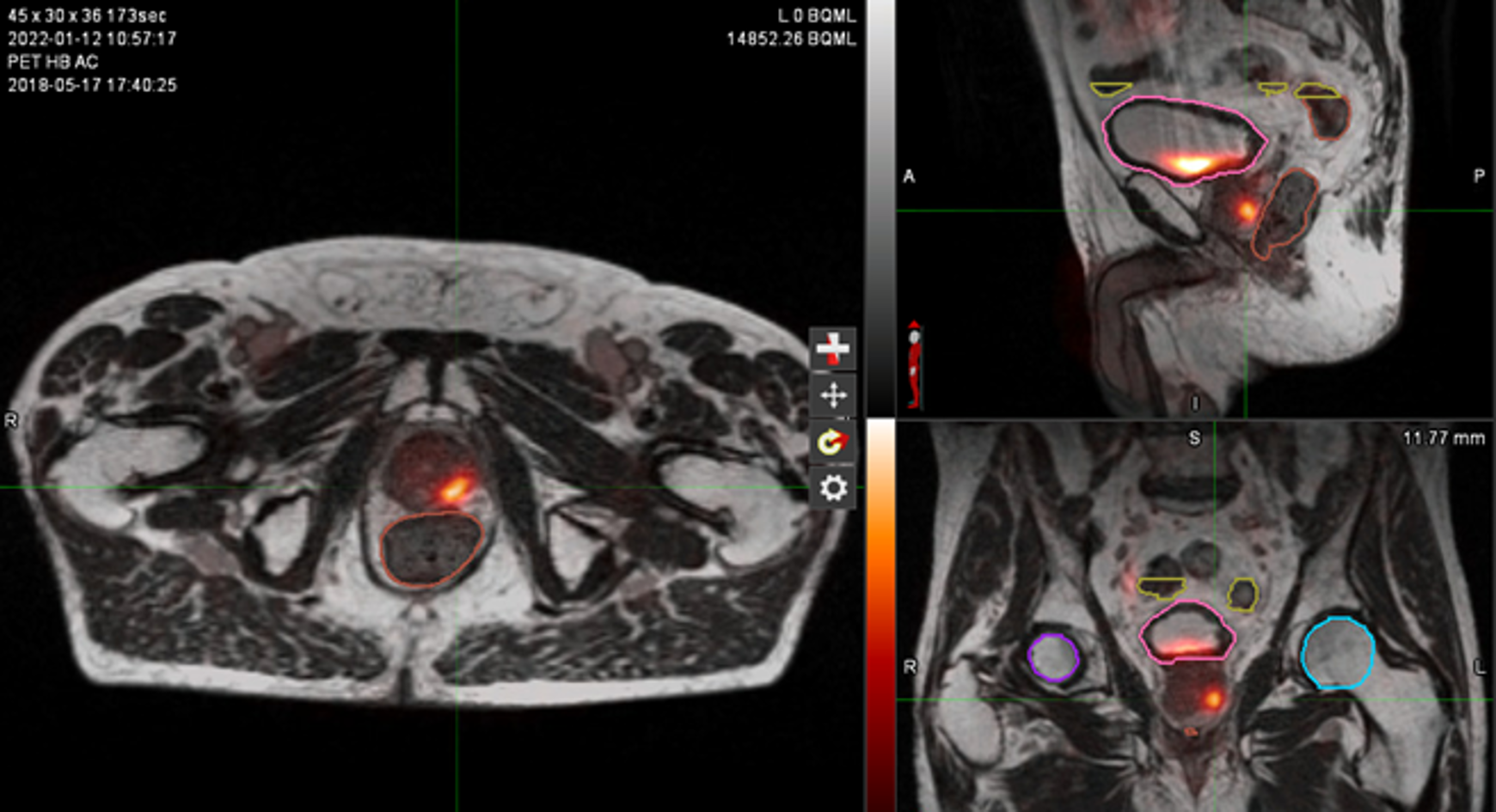
Figure 1: MR and PSMA PET fusion showing recurrence
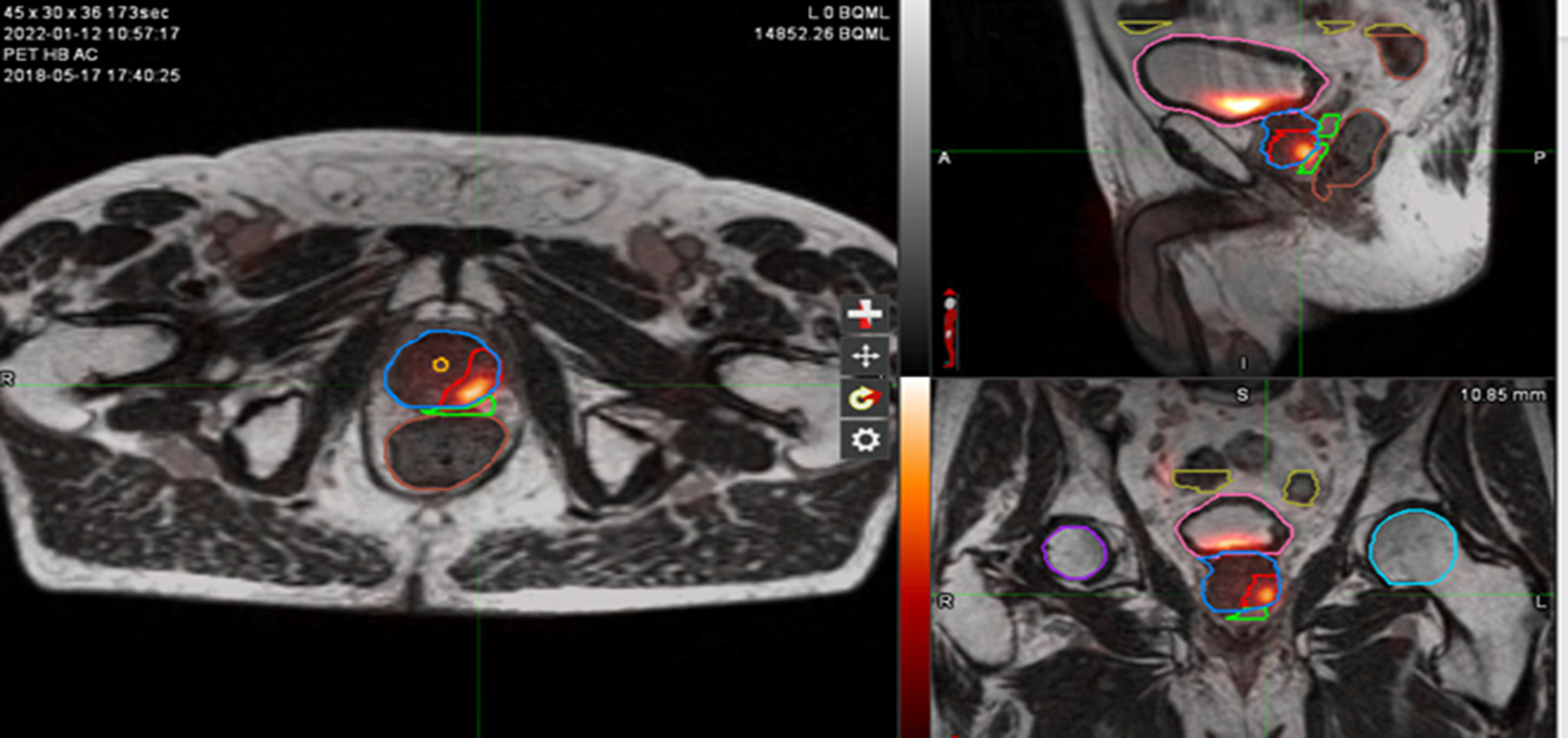
Figure 2: Contouring target lesion
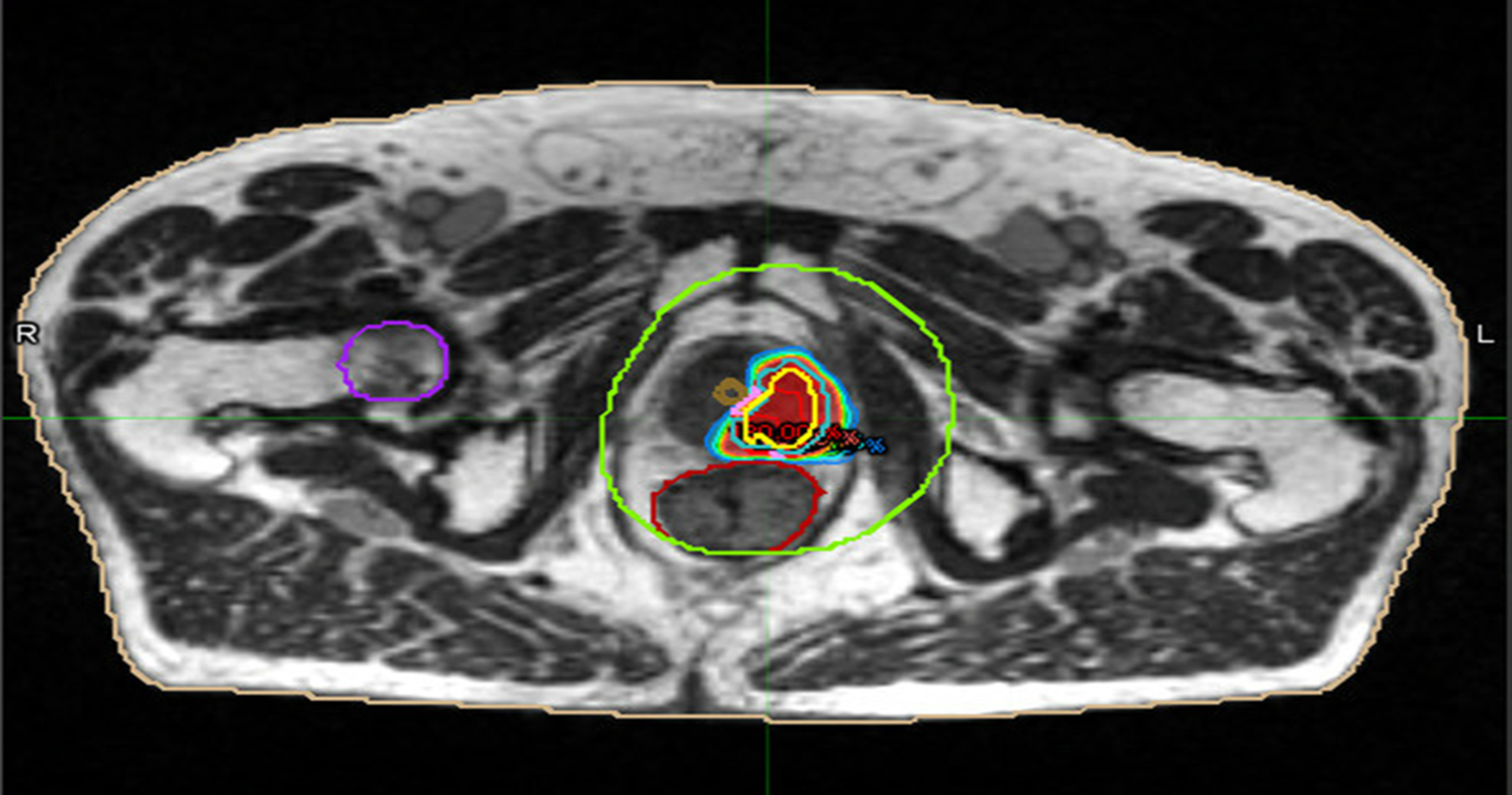
Figure 3: radiotherapy plan axial view
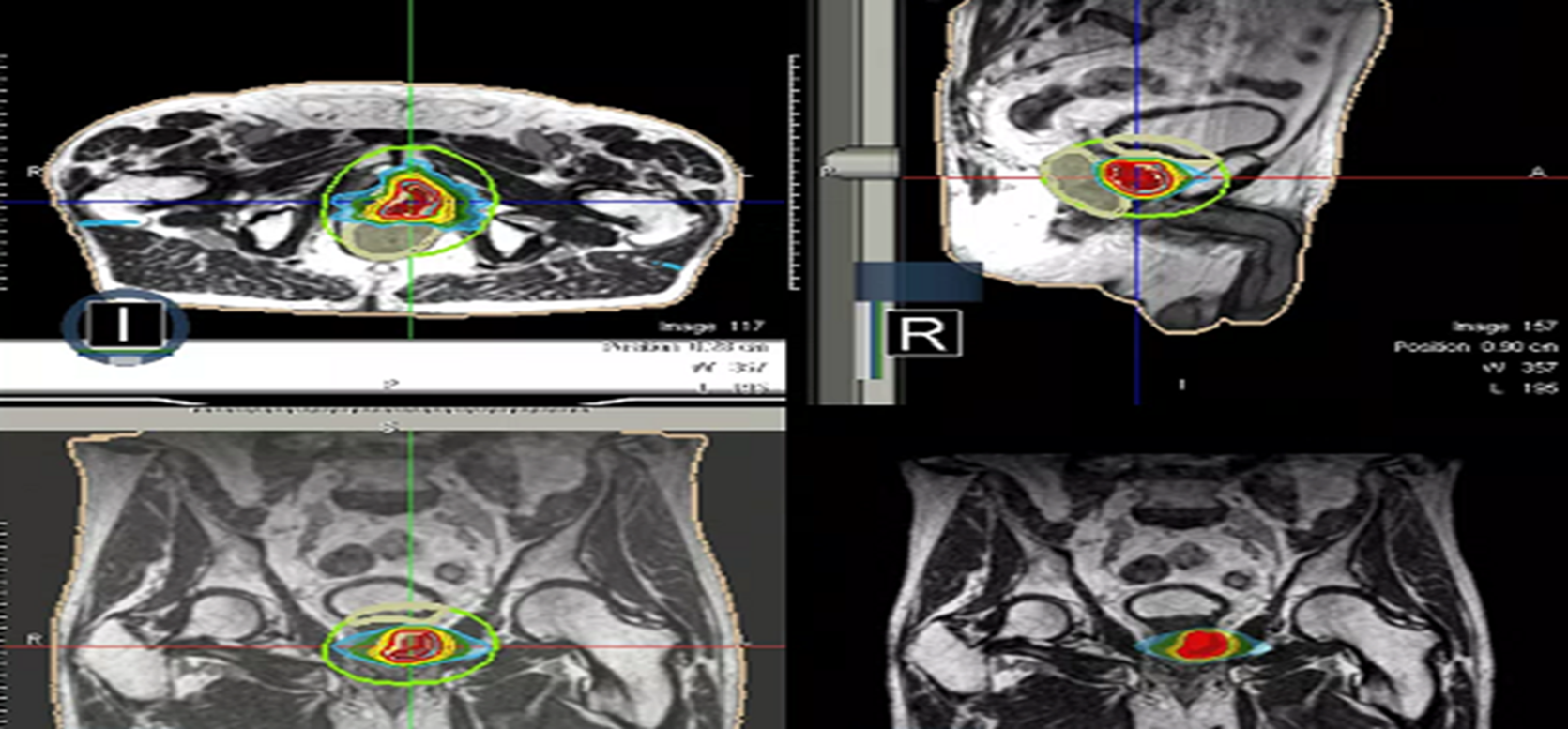
Figure 4: Radiotherapy plan 3D view
Discussion
After definitive radiation treatment of prostate cancer, local recurrence alone occurs in up to 30% cases. Less than 2% of patients undergo some form of local salvage therapy. Most patients receive androgen deprivation therapy (ADT) with attendant side effects. Due to superior radiotherapeutic ratio that allows dose escalation, while meeting the OARs dose constraints, MR-guided reirradiation (reRT) is an option to achieve safe local control, avoiding prolonged treatment with ADT.
This case demonstrated how re-irradiation is an attractive option for a patient to achieve excellent disease control without compromising QOL.
Speak to a MRIdian SABR expert
Our specialist can provide more information on MR-guided stereotactic radiotherapy at GenesisCare.
Prostate – methotrexate contraindication
Dr Ami Sabharwal

The benefit of MRI-guided radiotherapy to treat prostate cancer in a patient with rheumatoid arthritis on methotrexate
Case presentation
A 62-year-old male was diagnosed with localised, high risk prostate cancer in November 2019. The patient presented with a PSA level 8.87, stage T3aN0M0 with a Gleason score 3+4 = 7 (grade group 2). The gentleman had a long history of rheumatoid arthritis (RA), which was successfully treated with weekly methotrexate, alleviating all his RA symptoms.
An active semi-retired farmer, he was otherwise healthy and still assisting in farming activities including harvesting hay and felling trees for logs.
Challenges of presentation and choice of treatment
Treatment options were complicated by the fact that the patient had RA, which was being treated with methotrexate. Methotrexate is a radiosensitiser, increasing the toxic effects of radiotherapy.1 It was therefore discontinued four weeks prior to radiotherapy, for the duration of treatment and for four weeks following radiotherapy. There was concern that the patient’s RA would flare up over the three-month period he would need to be off treatment for conventional radiotherapy, equivalent to one month prior, one month during and one month post radiotherapy.
The patient was anxious that he would be unable to continue his farming activities during radiotherapy due to both the side effects of treatment and the potential worsening of his RA symptoms. This would obviously limit his physical activity.
A decision was made to treat on the MRIdian MR linac to deliver stereotactic ablative radiotherapy (SABR) to the prostate in five treatments (over 10 days), rather than the conventional 20 treatments, to minimise time off methotrexate and possibly avoid a flare-up of his RA. With this approach the total time off methotrexate would be maximally 10 weeks, versus a minimum of 12 weeks with conventional radiotherapy treatment.
MRIdian treatment
SABR treatment on the MRIdian was prescribed at 36.25 Gy in five fractions. The aim of treatment was curative.
At each session, daily adaptation was performed to account for changes in the position of the prostate and for variable bladder and bowel filling. Figure 1 and figure 2 illustrate the typical differences seen between the original planning MRIdian scan and that seen on the day of treatment due to day-to-day organ movement. Figure 3 and figure 4 illustrate how these movements were accommodated with the on-table plan adaptation.
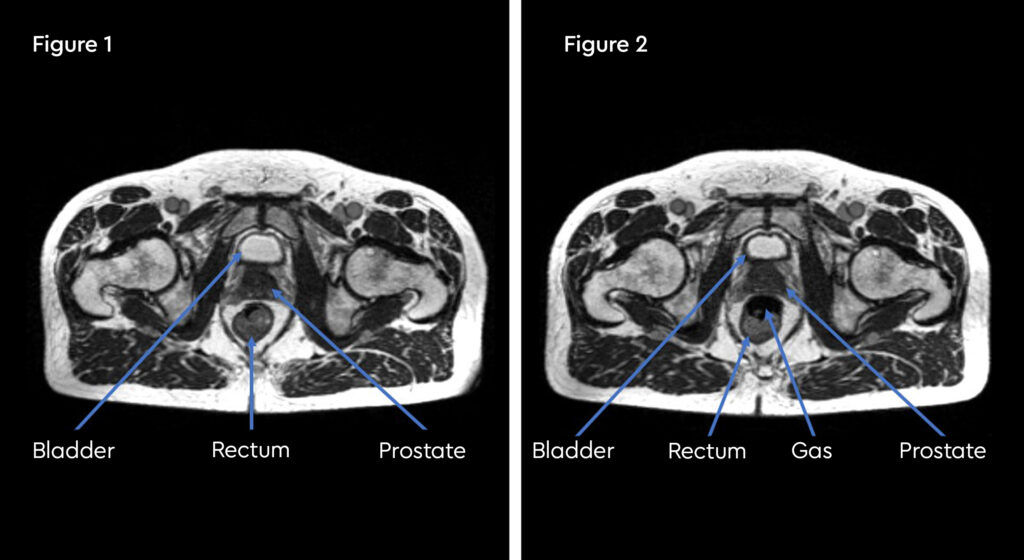
Fig 1: Original planning MRIdian scan
Fig 2: MRIdian scan at fraction 3, prostate position changed due to larger rectal diameter secondary to gas and smaller bladder
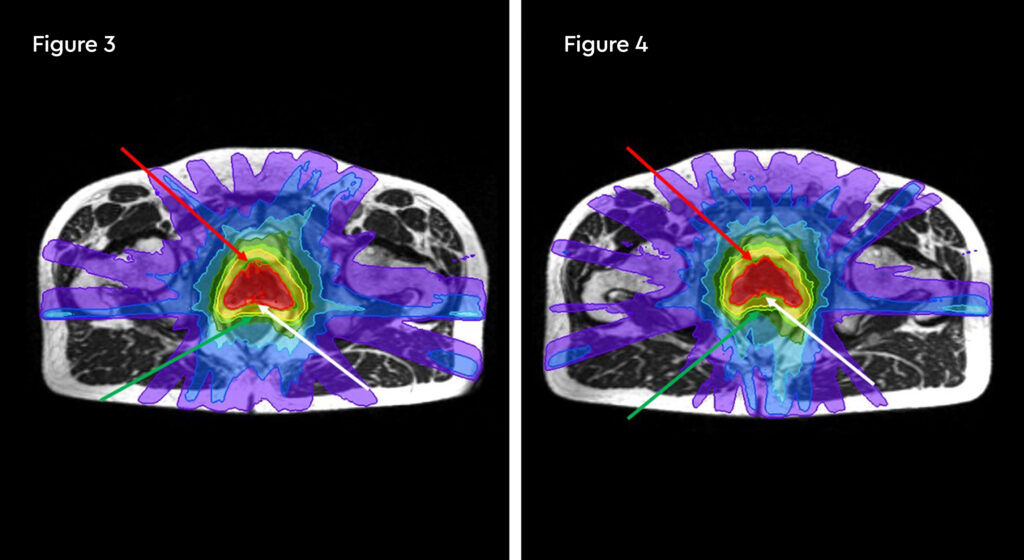
Fig 3: Prostate SABR delivery plan. Prostate volume covered by 95% isodose (green line, red arrow). Tight conformality at rectal/prostate boundary (white arrow), and rapid drop off of high dose (50% isodose, green line, green arrow) to optimise dose delivery to the prostate and limit dose to the rectum, reducing the risk of toxicity
Fig 4: Fraction 3, adapted plan, optimised for anatomy of the day. Prostate volume covered by 95% isodose (green line, red arrow). Tight conformality at rectal/prostate boundary (white arrow), and rapid drop off of high dose (50% isodose, green line, green arrow) to optimise dose delivery to the prostate and limit dose to the rectum, reducing the risk of toxicity
Each treatment including set-up and plan adaptation took 45 to 60 minutes during which time the patient was on the bed. As the patient was local to the treatment centre in Oxford he was easily able to attend the sessions and treatment was completed within two weeks.
Results and follow-up
The patient found the treatment process easier than expected; in fact, he commented that he was unsure if treatment had been delivered as he didn’t see or feel anything and experienced no significant bladder or bowel toxicities. Patient-reported outcome measures (PROMs) data collected showed no change with respect to bladder and bowel function between the start and end of radiotherapy. At the end of treatment, there was some improvement in tiredness and low mood reported.
At four weeks post-treatment toxicity check the patient reported urinary frequency (grade 1), but no other bladder or bowel symptoms. At the six-week post-radiotherapy follow-up, his PSA score was 0.09.
There was no deterioration of musculoskeletal function and no flare-up of the patient’s RA symptoms
Discussion
Delivering hypofractionated radiotherapy with the MRIdian reduced the length of treatment by two weeks which meant lesser disruption of methotrexate treatment for rheumatoid arthritis (RA). The patient was surprised at how well he tolerated treatment, allowing him to continue his normal farming activities even during and immediately following treatment without a flare-up of RA symptoms.
Prostate cancer treatment with MRIdian
A meta-analysis of stereotactic ablative radiotherapy (SABR) treatment in prostate cancer by Jackson et al in 2019, including 6116 patients, with a median follow-up of 39 months, demonstrated equivalent biochemical relapse-free survival, with conventional treatment.2
A phase 3 randomised control trial, HYPO-RT-PC, Windmark et al 2019, including 1200 patients, randomised to hypofractionated radiotherapy (42.7 Gy in seven fractions) versus conventional treatment (78 Gy in 39 fractions), demonstrated equivalent failure-free survival in both groups (84% in both groups, HR 1.002), with a median follow-up of five years.3
Prostate SABR on the MRIdian is associated with low levels of acute and medium term toxicity.
Low toxicity has been reported at one year following SABR on the MRIdian. In 101 patients, no severe (≥grade 3) toxicity was reported. No patients reported any limitation due to urinary symptoms; 97.8% of patients reported no limitation due to bowel symptoms.4
References
- Spittle, MF. Methotrexate and Radiation. Int J Radiat Oncol Biol Phys 1978 Jan-Feb;4(1-2):103-7.
- Jackson, W., Silva, J., Hartman, E., Dess, R., Kishan, A., Beeler, W., Stereotactic Body Radiation Therapy for Localized Prostate Cancer: A Systematic Review and Meta-Analysis of Over 6,000 Patients Treated On Prospective Studies. Int J Radiat Oncol Biol Phys. 2019;104(4):778-789.
- Windmark, A., Gunnlaugsson, A., Beckman, L., Thellenberg-Karlsson, C., Hoyer, M., Lagerlund, M., Ultra-hypofractionated Versus Conventionally Fractionated Radiotherapy for Prostate Cancer: 5-year Outcomes of the HYPO-RT-PC Randomised, Non-Inferiority, Phase 3 Trial. Lancet. 2019;394(10196):385-395.
- Tetar, S., Bruynzeel, A., Oei, S., Senan, S., Fraikin, T., Slotman, B., et al. Magnetic Resonance-guided Stereotactic Radiotherapy for Localized Prostate Cancer: Final Results on Patient-reported Outcomes of a Prospective Phase 2 Study. Eur Urol Oncol. 2020. https://doi.org/ 10.1016/j.euo.2020.05.7.
Prostate – metastatic recurrence
Dr Carla Perna

A case study of MRI-guided radiotherapy to treat a metastatic prostate cancer recurrence
Case presentation
A 58-year-old male with metastatic prostate cancer with recurrence in the left obturator nodes previously treated with external beam radiotherapy (EBRT). Due to the complexity of treatment, the patient underwent stereotactic ablative radiotherapy (SABR) using the MRIdian.
At initial diagnosis in 2015, the patient presented with a PSA value of 8, Gleason score 5+5 = 10 (grade group 5), stage T3aN0M0. He underwent radical prostatectomy and lymph node sampling, adjuvant radiotherapy to the prostate bed and androgen suppression.
In September 2017, he had a PSA rise to 0.68 and was found to have recurrent disease in the pelvic side wall, leading to these further lines of treatment and diagnosis over the next three years:
- Docetaxel chemotherapy
- Abiraterone
- A left hemi-pelvic salvage lymphadenectomy – due to recurrent disease with histology – which showed one lymph node with organised calcified fat necrosis and no sign of cancer
- Results of a CT-guided biopsy consistent with metastatic adenocarcinoma with neuroendocrine differentiation. This was then treated with three cycles of Carboplatin and Etoposide chemotherapy
- The patient then received five cycles of Lutetium177 PSMA therapy, which was discontinued in January 2020 due to disease progression. A PSMA PET scan completed after cycle five showed that the Lutetium PSMA had reduced the size of the recurrence and he was considered for SABR
The patient was an active gentleman, married with children and working as an engineer.
Challenges of presentation and choice of treatment
The patient had previously been treated with external beam radiotherapy (EBRT) to the prostate bed yet the cancer had recurred in the same area despite several lines of treatment. Although SABR was considered an option, the PSMA PET scan showed that the recurrence was close to organs at risk (OARs), particularly the rectum and the sacral nerve.
MRIdian MRI-guided radiotherapy was therefore chosen due to the complexity of the case and the difficulty of meeting the tolerance constraints of the OARs and of keeping toxicities within the mandatory limits.
MRIdian treatment
Stereotactic ablative radiotherapy (SABR) treatment with the MRIdian was prescribed at 30 Gy in five fractions, to the left obturator nodes. The aim of treatment was to ablate the single recurrence.
The SABR-COMET phase II multicentre trial investigated patients with oligometastatic disease from prostate, breast, lung and colorectum.1 It did show an increase in survival in patients with between one and five metastases. Median overall survival was 41 months for patients treated with SABR compared to 28 months in the standard treatment arm. Progression-free survival was 12 months in the SABR arm compared to six months in the standard radiotherapy arm.
Treatment took place over a two-week period in March 2020.
At each treatment session, on-table adaptation was performed to optimise the treatment delivery and spare the sacral nerve root. This process from set-up, contouring, plan adaptation and treatment delivery took approximately one hour for each session. The patient was able to travel from his home in Surrey for each appointment in Oxford.
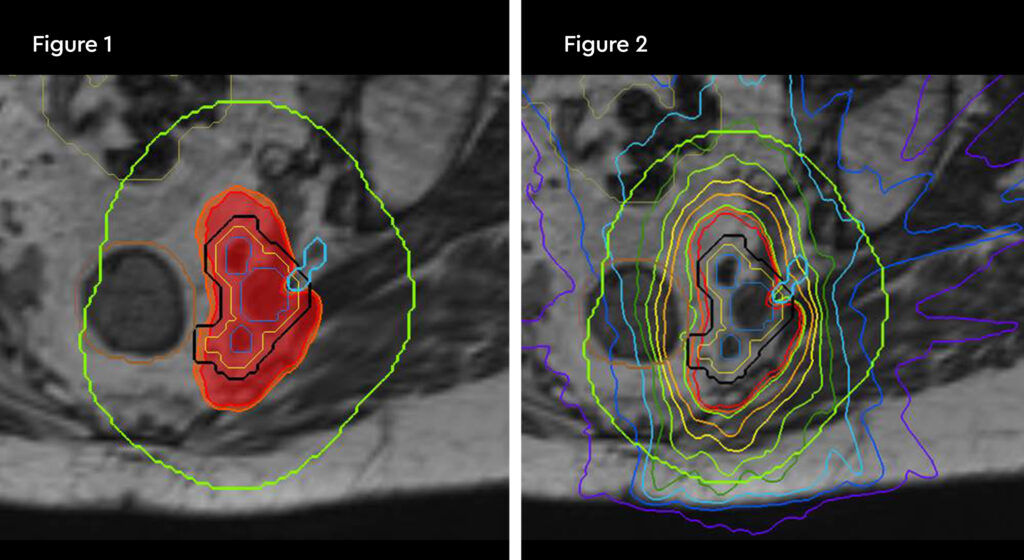
Fig 1 and 2: Planning. 100% isodose in red shaping off sacral nerve root, and orange contour is the rectum remaining tolerance dose of 28.8 Gy. Down to the 50% isodose in green
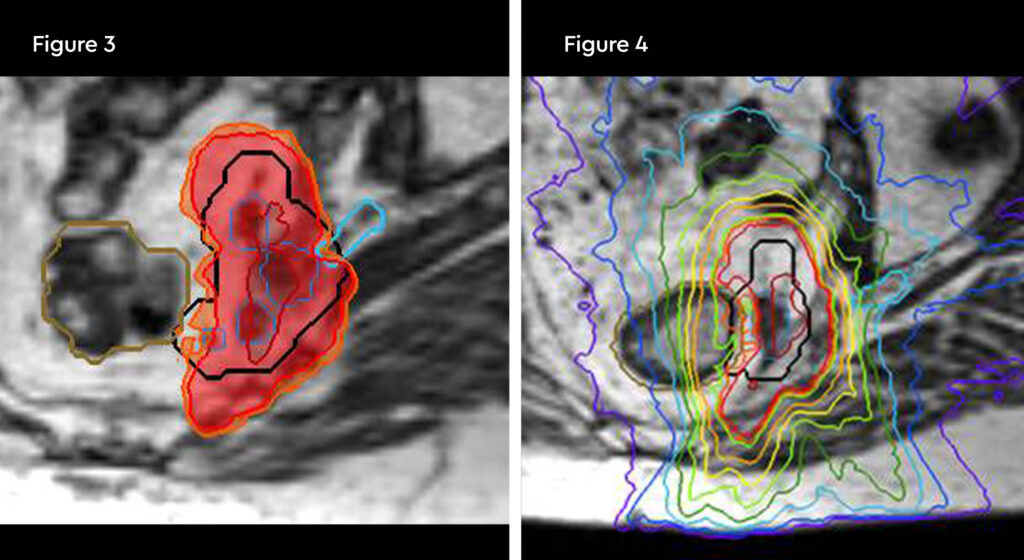
Fig 3 and 4: Fraction 1. Change in rectum position and 120% hotspot over the gross tumour volumes (GTVs)
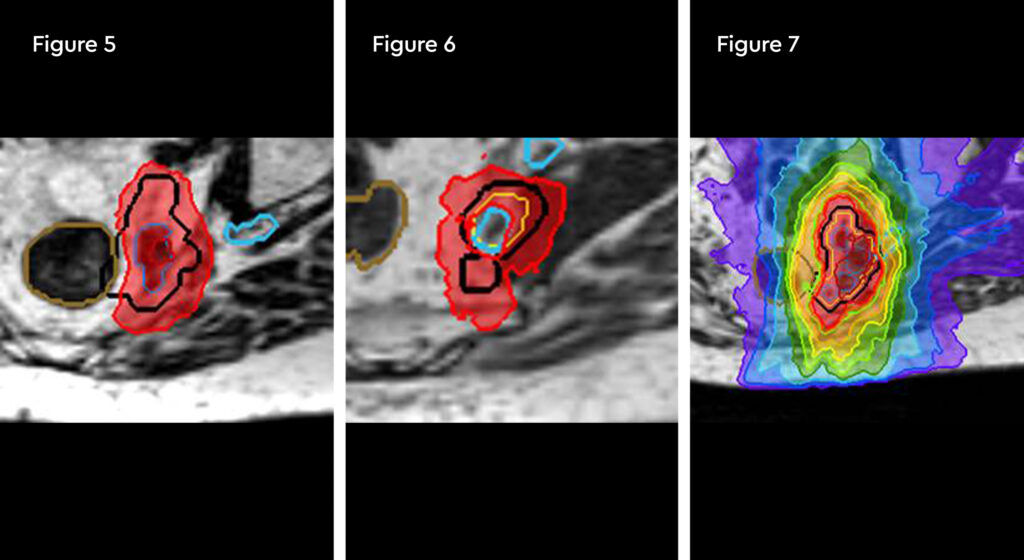
Fig 5: Fraction 3. Hot spot 120% directly in gross tumour volume (GTV)
Fig 6 and 7: Fraction 5. Nerve inside the planning target volume (PTV) being spared the 100% isodose
Results and follow-up
The patient tolerated the treatment well and reported no acute bowel toxicity or other side effects during or post radiotherapy. He was extremely happy with the outcome and also that the treatment has not impacted his quality of life.
At six-week follow-up the PSA value had markedly reduced to 0.11 and 0.3 from the baseline of 0.76 at 3 months follow-up showing a good response to treatment.
Patient-reported outcome measures (PROMs) data continue to be collected for this patient to monitor toxicities.
Discussion
This patient with metastatic disease had undergone most available modalities for prostate cancer and now had limited treatment opportunities for recurrence due to previous radiotherapy, and close proximity of organs of risk (OARs). Using MRIdian MRI-guided radiotherapy it was possible to use better tissue visualisation and live daily, on-table plan adaptation to limit toxicity and treatment aims were achieved with minimal impact to the patient’s quality of life.
References
- Palma, D., Olson, R., Harrow, S., Gaede, S., Louie, A., Haasbeek, C., et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393(10185):2051-2058.
Prostate – carer of his wife
Dr Prantik Das

A case study of MRI-guided radiotherapy to treat prostate cancer in a patient who was a main carer of his wife
Case presentation
An 82-year-old man with intermediate-risk localised prostate cancer was unable to undergo conventional radiotherapy due to family circumstances.
He was already under the care of a urologist in his hometown of Derby and diagnosed with stage T2cN0M0, a Gleason score 4+3 = 7 (grade group 3) and PSA value of 18. The patient had been started on bicalutamide but was struggling with the side effects of tiredness and painful enlargement of the breast. He also had a previous history of high cholesterol and was being treated with statins.
External beam radiotherapy (EBRT) was now being considered as a next line of treatment by his urologist.
A retired company owner, the gentleman is now a full-time carer for his wife who suffers from a chronic debilitating health condition.
Challenges of presentation and choice of treatment
Conventional EBRT radiotherapy for this patient would typically involve 20 to 37 fractions over four to seven and half weeks. The patient had decided against this treatment as he felt that travelling to hospital for several weeks would not be possible with no one to care for his wife. Instead, he had opted for long-term oral endocrine treatment, but this was now affecting his quality of life.
Stereotactic ablative radiotherapy (SABR) was then considered as a recommended treatment for low and intermediate-risk prostate cancer. Treatment can be completed in five fractions which would allow the patient to care for his wife.
The option to deliver this SABR on the MRIdian offered additional benefits. The daily adaption can allow for intra and inter fractional movement of the prostate and organs at risk (OARs), improving treatment accuracy and therefore reducing toxicity to healthy tissue. This could help to achieve a better quality of life (QoL) for this patient. It would also allow him to stop long-term endocrine treatment which was also adversely affecting his QoL.
MRIdian treatment
The patient was treated with five fractions of SABR using the MRIdian for MRI-guided treatment delivery, with the aim to achieve maximum disease control or be curative. Treatment commenced in December 2019 and was given on alternate days to meet the patient’s requested pre-Christmas completion date.
SABR treatment on the MRIdian was prescribed at 36.25 Gy in five fractions. During each treatment session, daily adaptation was performed to account for changes in the position of the prostate due to variable bladder and bowel filling. It has been well recognised that the prostate moves during the course of radiotherapy.
Figure 1 and figure 2 illustrate the typical interfraction movement as seen on the MRI scan. Figure 3 and figure 4 demonstrate the daily changes of the patient’s internal anatomy whilst figure 5 and figure 6 show the adaptive radiotherapy plan.
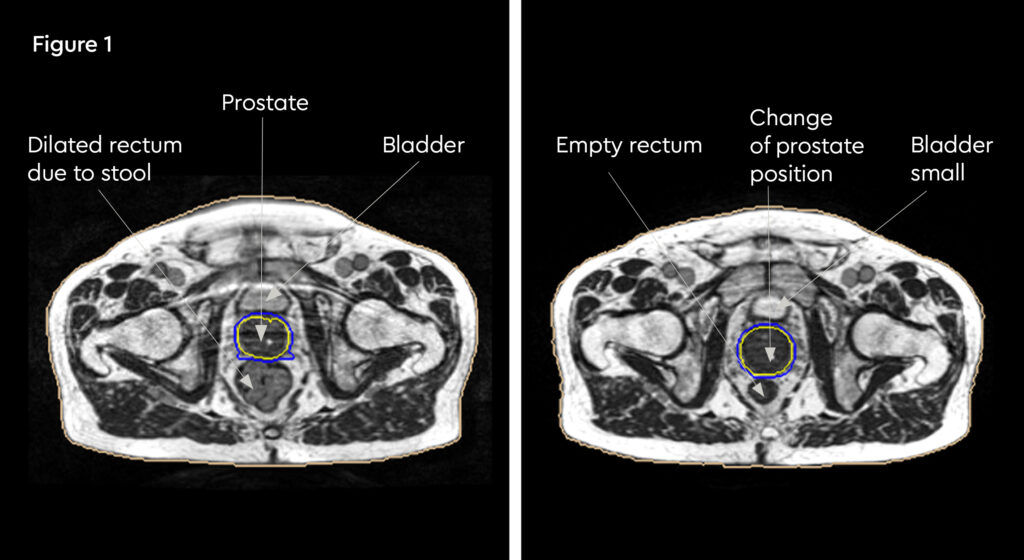
Fig 1: Internal anatomy during the planning MRI
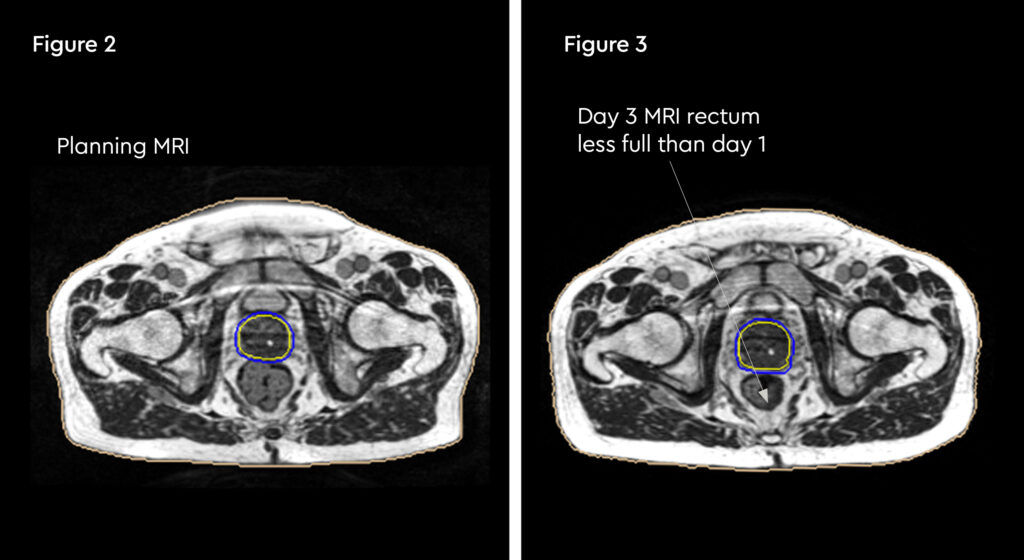
Fig 2: Internal anatomy during first fraction of treatment
Fig 3: Changes in internal anatomy demonstrated by a smaller rectum on day 3 compared to the planning scan
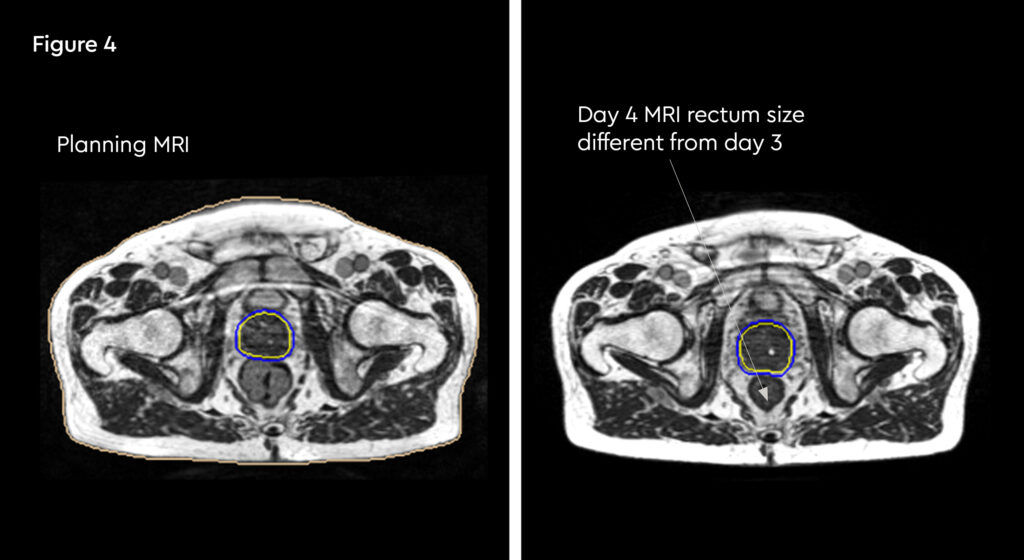
Fig 4: Changes in internal anatomy demonstrated by a different rectum size on day 4
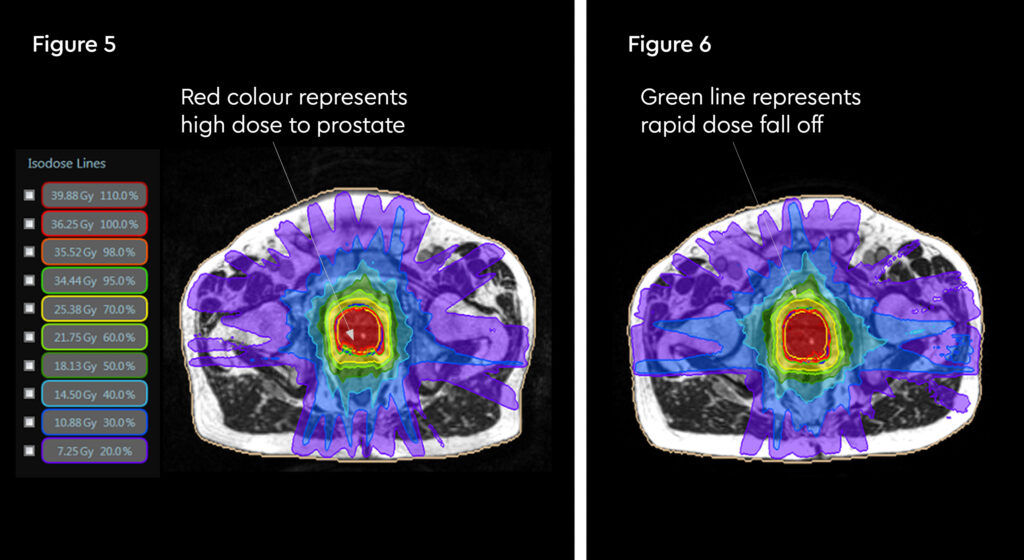
Fig 5: Radiotherapy plan
Fig 6: Plan adaptation. Tight conformality at rectal and prostate boundary is demonstrated by the arrow. Rapid reduction of high dose optimises dose delivery to the prostate and limit dose to the rectum, reducing the risk of long term toxicity.
Each treatment session, including set-up and plan adaptation, took 45 to 60 minutes during which time the patient was on the bed.
Although the patient lived in Derby, he was able to travel to the GenesisCare centre in Oxford each day for treatment and private transport was provided as part of his care.
At each session, the target was recontoured by the treating clinical oncologist and the plan adapted daily before treatment. This was found to be necessary due to the degree of movement and shifting of the prostate influenced by bowel and bladder filling.
This daily adaptive process took an average of 54 minutes for each of the five SABR treatment sessions.
Results and follow-up
Minimal side effects were recorded, with grade 1 bowel and grade 1 urinary toxicities which settled very quickly after treatment. In fact, these side effects were so minimal that the patient was concerned that treatment might not be effective.
Post-radiotherapy, his endocrine treatment was stopped, the side effects of tiredness had reduced within three weeks and he was feeling significantly better. At five months follow-up, the patient’s PSA value had decreased to 1.6 from 18 at the start of radiotherapy treatment.
The patient is continuing to provide patient-reported outcome measures (PROMs) data.
MRI-guided radiotherapy allows technology to be extended for use both in radiotherapy planning and for adaptations during treatment. The advantages of this are numerous – firstly, it can allow greater accuracy in delineating clinical target volumes and organs at risk.1,2 This in turn could lead to smaller volumes being irradiated which may reduce both acute and long-term radiation toxicity. Furthermore, rather than having a ‘snapshot’ of the anatomy, as would be provided by traditional IGRT (image-guided radiotherapy) such as a cone beam CT, this allows direct visualisation of the relevant area during the radiotherapy treatment.
The MRIdian MR linac enabled this patient to undergo curative radiotherapy treatment for prostate cancer with minimum impact on his day-to-day responsibilities as a carer for his wife. This was achieved with minimal short-term toxicities and in doing so, it was possible to reduce the side effects of long-term hormone treatments, greatly impacting the patient’s quality of life.
This gentleman was the first patient to be treated on the MRIdian at our GenesisCare centre in Oxford and this was also the first ever MRIdian treatment in the UK. We are delighted we could provide a positive outcome for him and his wife.
References:
- McPartlin, A., Li, X., Kershaw, L., Heide, U., Kerkmeijer, L., Lawton, C., et al. MRI-guided prostate adaptive radiotherapy – A systematic review. Radiotherapy and Oncology. 2016;119(3):371-380.
- Byrne, TE., A review of prostate motion with considerations for the treatment of prostate cancer. Med Dosim 2005;30:155–61.
- Schild, S., Casale, H., Bellefontaine, L., Movements of the Prostate Due to Rectal and Bladder Distension: Implications for Radiotherapy Med Dosim Spring 1993;18(1):13-5.
Prostate – with a prosthetic hip
Dr Nicola Dallas

A case study of MRI-guided radiotherapy to treat prostate cancer in a gentleman with a left prosthetic hip
Case presentation
A 74-year-old male with intermediate risk prostate cancer. The patient had a PSA value of 9.8 and a multiparametric MRI scan revealed a left peripheral zone tumour at the apex of the prostate along with the known previous left total hip replacement.
The patient was subsequently diagnosed with a prostate adenocarcinoma stage T2bN0M0 with a Gleason score 3+4 = 7 (grade group 2). He was started on bicalutamide hormone treatment. At the time of presentation, he was also taking tamsulosin to reduce his urinary tract symptoms.
Despite the hip replacement this gentleman remained very active – walking and running daily and continuing to work.
Challenges of presentation and choice of treatment
A hip replacement ordinarily produces significant artefact on a planning CT and diagnostic MRI. This presents particular challenges for conventional radiotherapy, making it difficult to visualise and contour the prostate gland for radiotherapy planning. This inability to accurately define the prostate gland in turn increases the potential toxicity to surrounding structures during treatment.
Fig 1: Planning scans of the patient. The top image shows MRI planning images using 0.35 T MRIdian displaying minimal artefact around the left prosthetic hip. The bottom is a CT planning scan of same patient showing significant artefact and obscured prostate views
Prostate brachytherapy was not considered a suitable treatment alternative to external beam radiotherapy here because of this gentleman’s significant lower urinary tract symptoms, and consequent use of tamsulosin. Brachytherapy can cause a significant and prolonged flare of genitourinary symptoms, and possible urinary retention, in patients with poor functional parameters pre-treatment.
Experience in other centres, such as the VU University Medical Center (VUMC) in Amsterdam, had already demonstrated that a hip prosthesis produces minimal interference on the imaging function of the MRIdian MR linac, enabling accurate localisation of the prostate gland. The MR linac technique then combines daily adaptation and real-time visualisation to facilitate a significantly reduced treatment margin, thereby minimising rectal dose and associated toxicity.
The patient therefore opted for treatment with ultra hypofractionated radiotherapy on the MR linac, on the basis that with improved localisation of the prostate it could minimise potential toxicity and long-term side effects to his rectum and bladder.
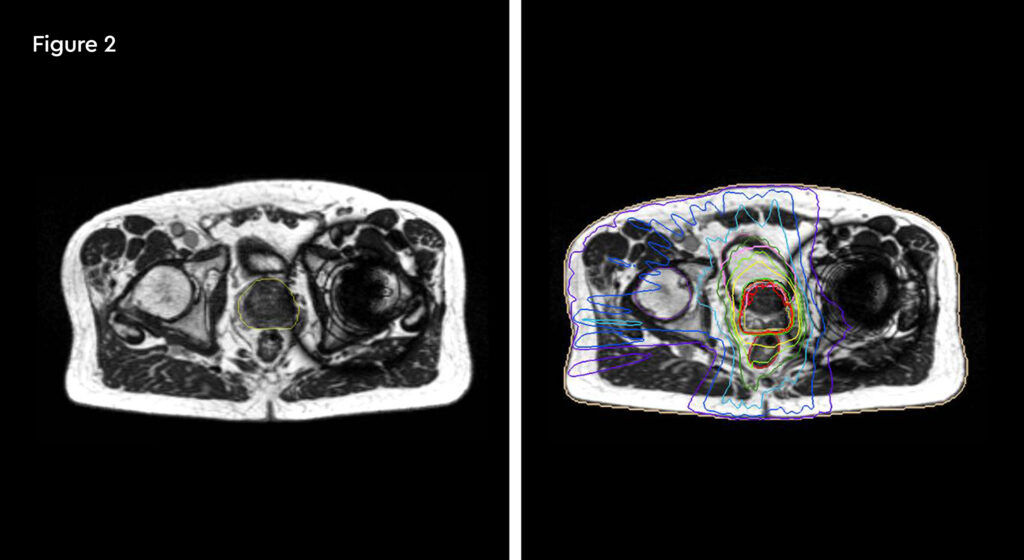
Fig 2: The MRIdian treatment plan with prostate contour on the planning MRI scan shown on the left and the prostate plan achieved on the right
MRIdian treatment
Treatment was prescribed at 36.25 Gy in five fractions on the MRIdian. The aim of treatment was curative.
Treatment commenced within two weeks and was delivered in five fractions over two weeks. During this time, the patient travelled to each fraction from his home in Birmingham. At each session, minimal adjustments were made to the clinical target volume and the organs at risk (OARs) were recontoured for their daily position. Most fractions were completed within a 40-minute timeframe including patient set-up.
In the event, the imaging quality of the MRIdian’s in-built MRI function proved to be excellent, facilitating accurate organ visualisation and targeting. The patient, complete with prosthetic hip tolerated treatment on the MR linac, confirming the experience of other centres.
Results and follow-up
At the time of writing this case study, it was too soon to assess side effects and treatment outcome.
The patient will complete patient reported outcome measures (PROMs) questionnaires, but at the time of writing he has tolerated the treatment well and reported no significant impact on lifestyle, other than travelling to and from treatment.
Discussion
Radiotherapy treatment to the prostate gland is possible for patients who have a hip prosthesis using the MRIdian MR linac. This technique provides high quality visualisation of the prostate and is well tolerated. Future data will be reviewed to measure the full benefit of this therapy in reducing toxicities and improving quality of life.
Sub-diaphragmatic node in the upper abdomen
Dr Philip Camilleri

A case study of MRI-guided radiotherapy to a sub-diaphragmatic node in the upper abdomen
Case presentation
A 52-year-old female with metastatic renal carcinoma. At the time of referral for stereotactic ablative radiotherapy (SABR) she had oligoprogressive disease in a single lymph node beneath the right diaphragmatic crus (figure 1).
The patient was initially diagnosed in October 2018 when she also underwent an open right radical nephrectomy confirming the presence of renal clear cell carcinoma with rhabdoid change, stage T3bNxMx. Further investigation revealed the presence of multiple pulmonary metastases and she was commenced on nivolumab and ipilimumab immunotherapy treatment. Her metastatic disease had been completely controlled with no evidence of growth until a routine check CT scan identified an enlarging right upper abdominal node adjacent to the right diaphragmatic crus. At the same time, she developed right upper abdominal pain which was thought to possibly be due to the nodal growth but also possibly due to gallstones which she was also known to have.
She was otherwise enjoying an active lifestyle which had been mostly unchanged by her disease.
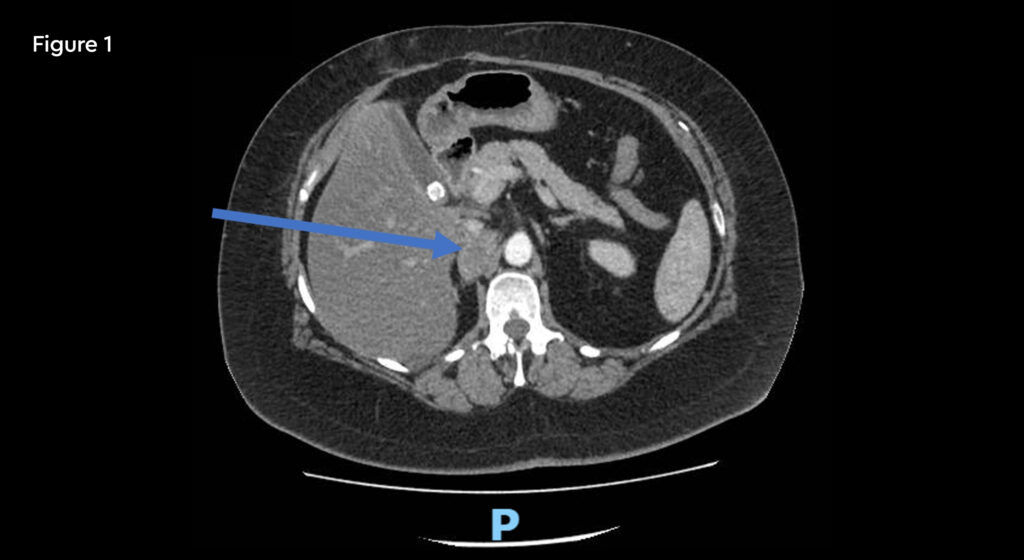
Fig 1: Pre-treatment CT scan showing right upper abdominal node measuring 25mm on 10th January 2020
Challenges of presentation and choice of treatment
Radiotherapy was an option to achieve local disease control. However, this was a complex case due to the close proximity of the liver and small bowel, and the movement of these organs at risk (OARs) with respiration.
The opportunity to treat this patient with SABR using the MRIdian MRI-guided system was reviewed with the SABR Advisory Team at GenesisCare.
With its integral MR scanner and real-time imaging, MRIdian offers the capability of good soft tissue visualisation in the upper abdomen as well as allowing clinicians to account for the location of small bowel and other significant OARs, adapting the radiotherapy plan on a daily basis. This adaptive radiotherapy, together with the breath-hold and automated beam gating function of MRIdian, allows a high dose to be safely delivered to the target.
MRIdian treatment
Stereotactic ablative radiotherapy (SABR) treatment on the MRIdian MR linac was prescribed at 40 Gy in three fractions. Treatment sessions were carried out on alternate days and were completed at the end of February.
The dose prescribed was in line with the UK SABR consortium guidance for stereotactic radiotherapy to involved nodes.
At each session the plan was recontoured and the daily plan was adapted to match the position of the target node as well as the surrounding organs at risk, specifically the small bowel and liver. This was important to ensure the dose to organs at risk (OARs) was kept to an absolute minimum. The patient was also required to use the breath-hold visualisation, to enable the automated beam gating. Each treatment session took approximately 45 minutes, including contouring and plan adjustment.
Results and follow-up
The patient noted very few treatment side effects. She had grade 1 fatigue lasting two weeks and no nausea, vomiting or bowel disturbance.
Patient-reported outcome measures (PROMs) are collected from baseline and show that although she had recorded moderate fatigue prior to her SABR treatment and on the day of her final fraction, four weeks and three months post treatment she recorded no fatigue.
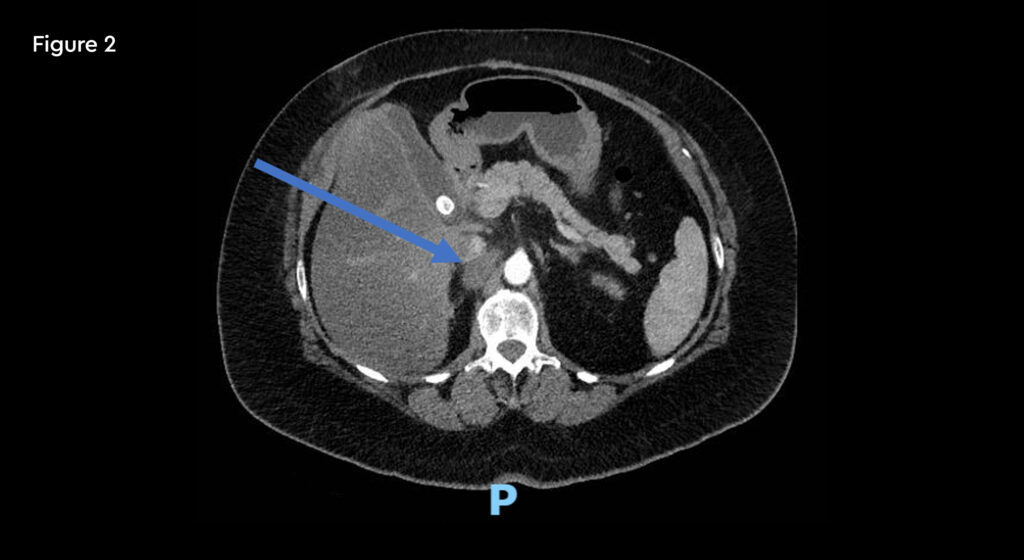
Fig 2: Post-treatment CT scan (19mm) on 12th May 2020 10 weeks after completion of SABR
Other PROMs show no change from baseline in pain, gastric symptoms, intestinal symptoms, sexual functions or emotions.
A follow-up CT scan at 10 weeks after SABR treatment showed a reduction in size of the node.
Discussion
SABR using the MRIdian MR linac allowed safe delivery of a high dose, while limiting toxicity to OARs. In the event, this was found to be a straightforward treatment approach. The patient was delighted to have completed her treatment so quickly and with almost no effects apart from mild fatigue.
Multiple liver metastases
Dr James Good

A case study of MRI-guided radiotherapy to treat multiple liver metastases
Case presentation
A 64-year-old lady with a history of HER2 negative ER+ breast cancer. She initially presented with stage pT2N1M0 disease and had previously undergone wide local excision and post-op radiotherapy, chemotherapy and endocrine therapy. She relapsed with three liver metastases on FDG PET and MRI and on the basis of the SABR COMET trial results, and a preference to avoid surgery, was referred for stereotactic ablative radiotherapy (SABR).
Challenges of presentation and choice of treatment
The challenge here was the number of metastases. Two of them were in a position not amenable to percutaneous radiofrequency ablation, and given that breast cancer can recur in other tissues as well as liver, surgery was likely not to be the optimal approach. The lesions were positioned in different segments of the liver, making it challenging to treat with conventional SABR due to the need for an internal target volume (ITV), leading to an increased mean liver radiation dose and therefore potentially reduced dose to the tumours.
MRIdian treatment
The patient accepted treatment on the MR linac despite being apprehensive about the treatment time. The lesions were readily visible on the MRIdian planning scans with IV liver-specific contrast (figure 1). She completed treatment as planned, and we were able to deliver 50 Gy in five fractions to all three lesions. During the adaptive replanning process we could see that the gross tumour volumes (GTVs) were decreasing in size as SABR proceeded. She has also continued with second line endocrine therapy.
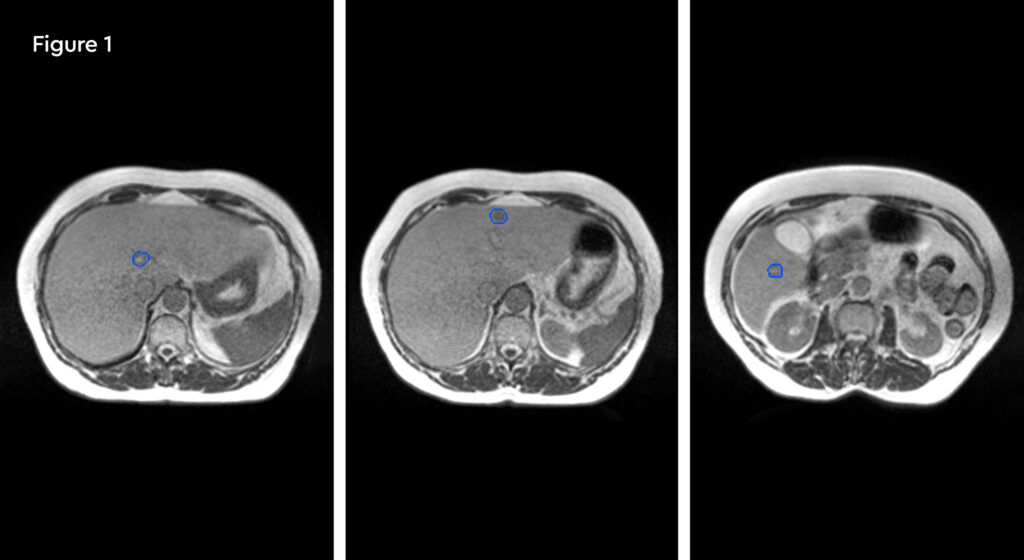
Fig 1: Pre-treatment MRIdian planning scans showing three small, scattered liver metastases
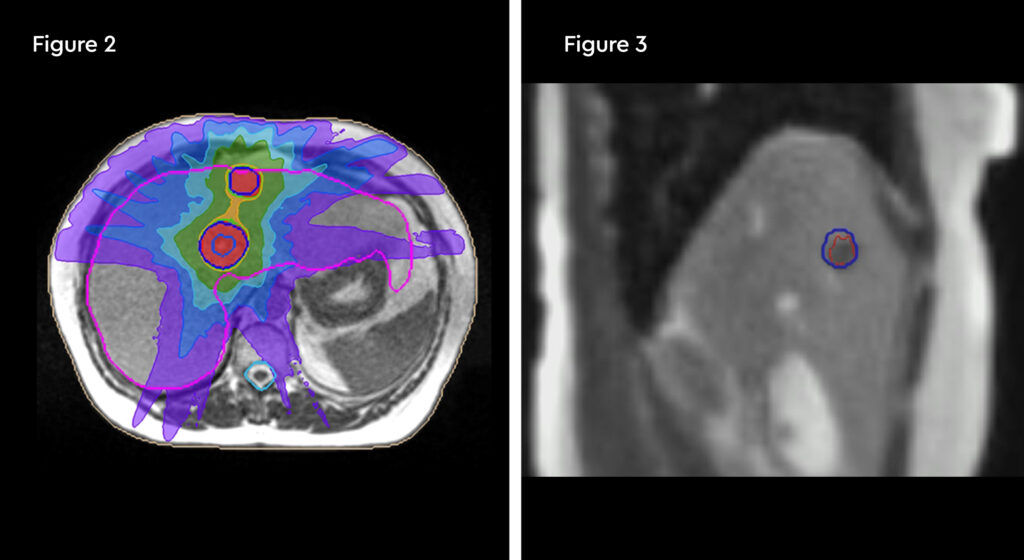
Fig 2: MRIdian SABR plan, showing tight dose distributions across two of the three tumours
Fig 3: Treatment tracking image, which empowered the patient to participate in her own treatment
Results and follow-up
The patient did develop some rib tenderness related to radiation dose to the most peripheral lesion, but this settled with simple analgesia and time. MRI scan at three months showed the tumours were almost completely clear and a further scan at six months showed no active cancer. The patient requires ongoing endocrine therapy to control her disease.
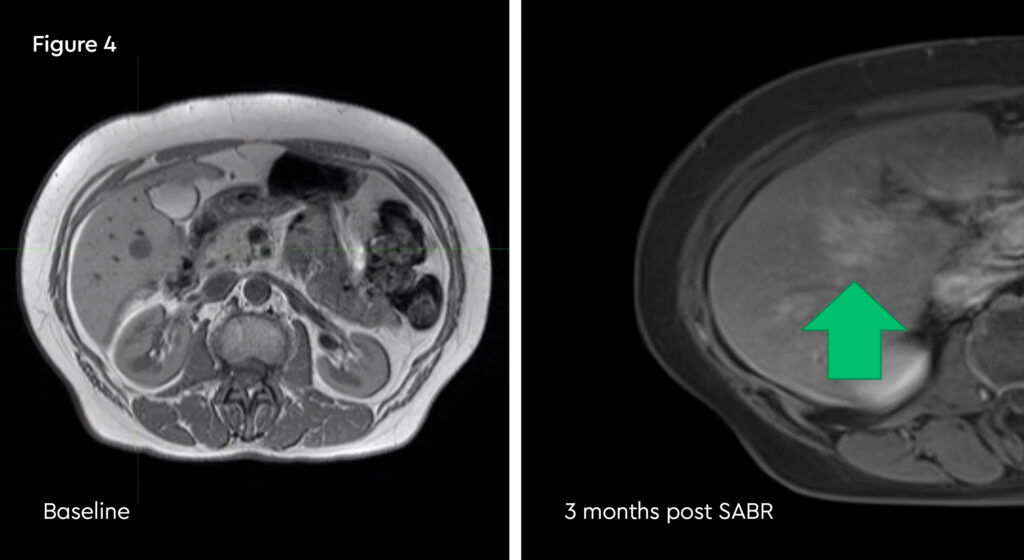
Fig 4: Post-treatment MRI scan, showing a well demarcated ablation zone
Discussion
The improved soft tissue definition, beam gating and adaptive replanning made possible by MRIdian allowed an entirely non-invasive approach to ablating this lady’s liver metastases. MRI-guidance added value through greater confidence in delineating and tracking gross tumour volumes smaller treatment margins and maximising the ablative radiation dose.
Pancreatic tumour
Dr Andy Gaya

A case study of MRI-guided radiotherapy to treat a pancreatic tumour
Case presentation
A 47-year-old female with oligometastatic disease and a local recurrence of pancreatic ductal adenocarcinoma, adjacent to superior mesenteric vessels.
The patient was originally diagnosed in 2018 with moderately differentiated disease in the uncinate process, which was borderline resectable and in close proximity to the superior mesenteric vein. She presented with obstructive jaundice in late June 2018 and was treated with a metallic stent inserted to the central bile duct. Neoadjuvant Folfirinox chemotherapy commenced in July with stable disease after four cycles. This was followed by chemoradiotherapy to the uncinate process tumour and subsequently a Whipples resection and partial gastrectomy at the end of December that year. This identified poorly differentiated (grade 3) adenocarcinoma, stage T2N1M0 with R1 SMA margin, the positive margin was clipped and adjuvant Folfirinox was commenced from February to May 2019.
In September 2019 there was a local recurrence adjacent to the superior mesenteric vessels and the patient was given gemcitabine and nab-paclitaxel chemotherapy, with a partial response.
The patient was fit and healthy with two small children. A social smoker, she drank alcohol in moderation and had been diabetic since the onset of cancer to her pancreas.
Challenges of presentation and choice of treatment
This was a difficult case of incurable disease in a young woman with a young family. The pancreatic tumour was initially inoperable and then recurred within months of surgery.
The patient had received previous radiotherapy to the pancreas and therefore the surrounding normal tissues were already near tolerance. The only option for retreatment with a meaningful biological effective dose (BED) would be to use the MRIdian, which would accurately account for the varying position of the bowel and duodenum each day. This would be achieved by adapting the treatment plan at each session and using the respiratory beam gating during treatment.
MRIdian treatment
SABR treatment on the MRIdian was prescribed at 40 Gy in five fractions, with the aim of achieving local control. This was completed at the end of January 2020.
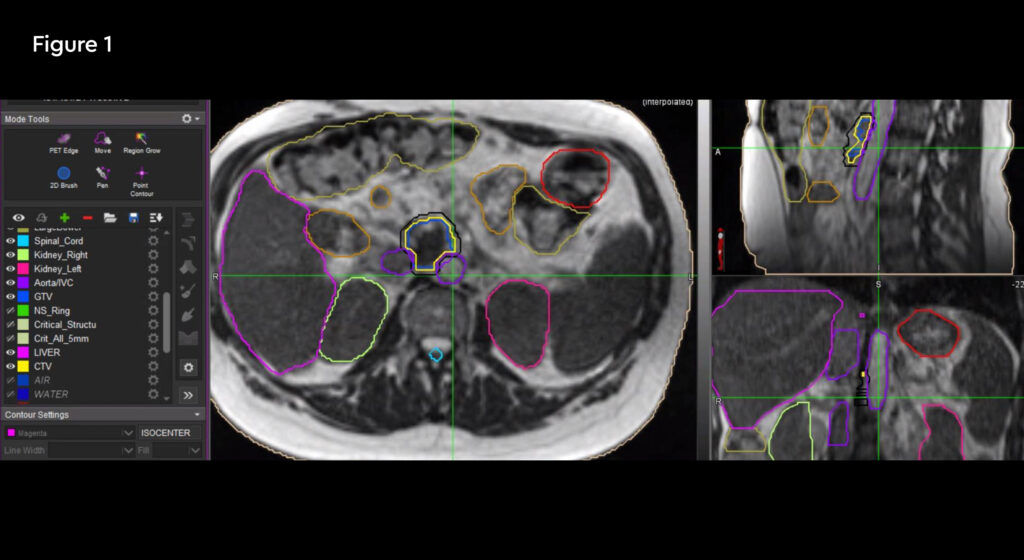
Fig 1: Anatomical location of the recurrent tumour and the critical organs at risk
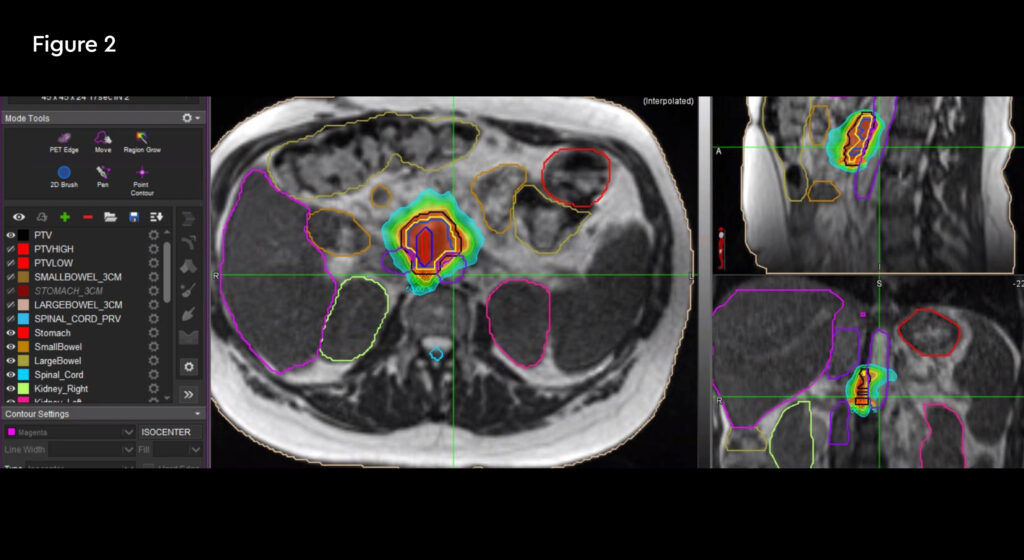
Fig 2: Radiation treatment plan down to 50% isodose
Treatment commenced within two weeks and was delivered over five days over a two-week treatment period. The patient travelled from her home in Hertfordshire for daily treatment. At each session the target and organs at risk (OARs) were recontoured and the plan was optimised to minimise dose to normal tissues. Due to complexity, treatment took 90 – 120 minutes to complete from set-up, plan adaptation and treatment delivery, during which time the patient remained on the bed.
She was also required to participate in the breath-hold function of MRIdian, essential for automatic beam gating to achieve smaller margins.
In March, the patient was re-commenced on chemotherapy with gemcitabine and nab-paclitaxel, which was completed at the end of April.
Results and follow-up
The patient tolerated her treatment well, with only some side effects; fatigue (grade 1), nausea (grade 2) and vomiting (grade 1). Overall she found the treatment had minimal impact on her lifestyle and was very happy with all aspects of her care.
She found the breath-hold visualisation in particular very empowering.
Patient-reported outcome measures (PROMs) data continue to be collected.
In June, coeliac and left supraclavicular (SCF) node biopsies were performed and revealed a new node in the SCF and porta hepatitis, which unfortunately showed distance progression. She is now receiving systemic therapies.
Discussion
Retreatment radiotherapy, to a full dose, even in difficult areas like the pancreas is possible with the MRIdian MR linac. For this patient, there was an opportunity to target local disease control when treatment options are limited, with minimal impact on her lifestyle.
Central lung tumour
Dr Veni Ezhil

A case study of MRI-guided radiotherapy to treat a central lung tumour
Case presentation
A 72-year-old male with a local recurrence of small cell lung cancer, five years after initial diagnosis. The patient was diagnosed in May 2015 with T3N1M0 limited stage small cell lung cancer and underwent systemic chemotherapy with six cycles of carboplatin and of etoposide with partial response. He was also given radical thoracic radiotherapy, 64 Gy in 32 fractions and prophylactic cranial irradiation, 25 Gy in 10 fractions.
In December 2019, a routine chest CT scan showed an area of relapse in the right upper lobe (RUL). Staging with PET confirmed a 1.8cm area of relapse in the RUL with no mediastinal nodes or metastatic disease. MRI brain screening was negative. A biopsy confirmed small cell lung cancer. He was re-challenged with four cycles of carboplatin and etoposide completed in February 2020. A PET scan showed stable disease and no evidence of new disease elsewhere.
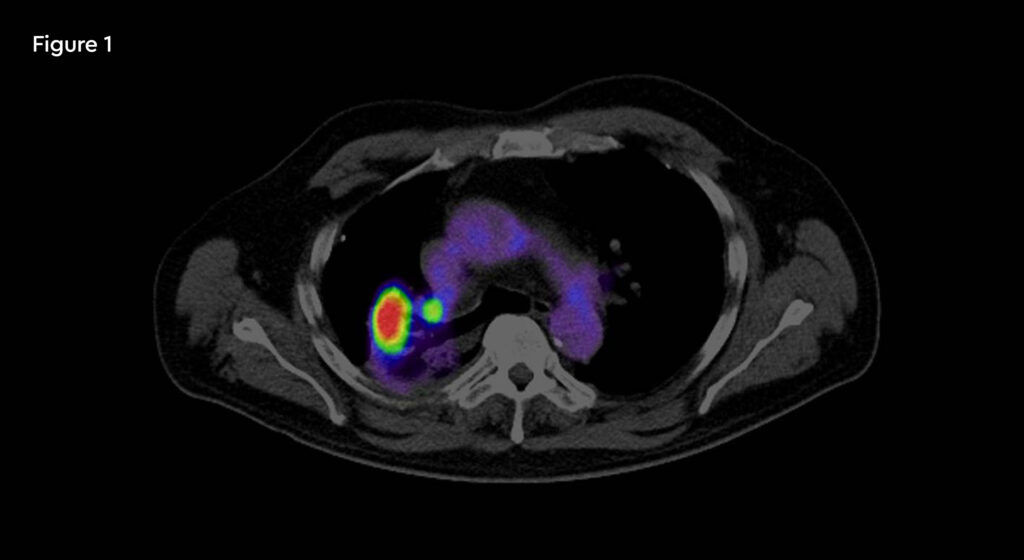
Fig 1: PET/CT image of recurrent tumour in the right lung
The patient, a retired fireman, was otherwise very fit with a regular exercise route and enjoyed long distance motorcycle rides.
Challenges of presentation and choice of treatment
Local relapse of a small cell lung cancer five years after diagnosis, with no metastatic disease. It was especially challenging due to the central location of the tumour and limitations for thoracic reirradiation of an in-field relapse.
Several treatment options were reviewed for this patient. These included:
- Surgery involving a pneumonectomy to ensure a complete resection. The risks of this approach included the potential complications of a major surgical procedure, loss of lung capacity in a fit and active person and the added risk of COVID-19 (at the time of treatment in early 2020)
- Reirradiation on a conventional linac, either as 45 Gy in 15 daily fractions or 45 Gy in 30 twice-daily fractions. Less biological effective dose (BED), compared with SABR. Also involved more clinic visits and risk to the patient during the COVID-19 pandemic
- Stereotactic ablative radiotherapy (SABR) on an MR linac with the aim to deliver a higher BED to maximise local control. The additional features of the MRIdian MR linac would ensure a smaller treatment volume due to gated breath-hold delivery. Daily recontouring would account for changes in position of the target and OARs and plan adaptation would aim for good target coverage, keeping all OARs in tolerance with every fraction. These combined to deliver lower toxicity 1,2
For these reasons the selected treatment option was SABR on the MRIdian MR linac, with a prescribed dose of 50 Gy in eight fractions. Treatment aims were to maximise local control at the only site of relapse, and reduce the risk of further disease progression.
MRIdian treatment
The patient started treatment in March within two weeks and received eight treatments delivered on alternate days. The patient travelled from Southampton to Oxford for each treatment. At each session, the target and organs at risk (OARs) were recontoured and the plan re-optimised prior to the fraction being delivered.
In the event, the set-up time took approximately seven minutes. The contouring took an average 17 minutes to complete, including the target and OARs and applying the required Boolean operations and rules. It took a further six minutes to check and preview the treatment area.
Treatment delivery itself took around 23 minutes including the gating, when the MRIdian automatically interrupted the beam to avoid OARs or if the target moved out of field. The patient was required to participate with the breath-hold delivery, which he tolerated very well, enabling a shorter time spent on the bed.
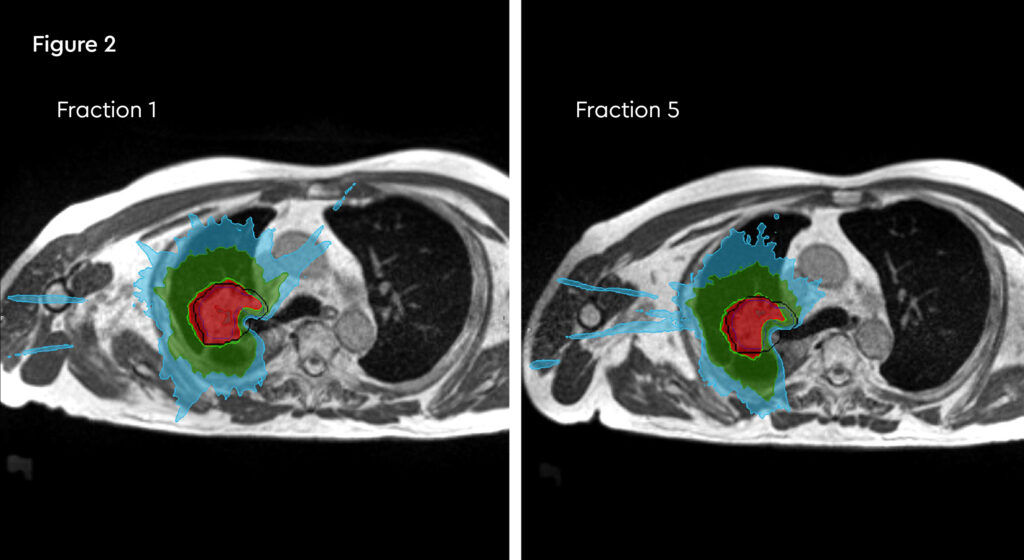
Fig 2: Daily re-contouring of a patient with central lung tumour. MRI scans from fraction 1 and fraction 5 show the extent of movement and variation and subsequent re-planning during adaptive treatment
Results and follow-up
The patient tolerated the treatment well with no acute toxicity, aside from grade 1 fatigue. He remained well at the four-week review and reported no toxicity. Patient-reported outcome measures (PROMs) recorded at baseline and at the end of treatment have indicated no side effects and the patient continues to participate in PROMs data collection.
A PET/CT scan in July was delayed as the patient had a fall.
Further follow-up CT scans are scheduled at three-monthly intervals to monitor disease relapse and any evidence of late toxicity.
Discussion
The MRIdian MR linac enabled complex radiotherapy involving a central lung tumour and re-irradiation of a field. Early feedback shows this was achieved with limited toxicity. Local disease control and the effects on disease progression will now be closely monitored to see if the higher biological effective dose (BED) has an effect on outcome.
The patient was able to tolerate the treatment and breath-hold feature particularly well, possibly because he was fit and active.
References:
- Finazzi, T., Haasbeek, C., Spoelstra, F., Palacios, M., Admiraal, M., Bruynzeel, A., et al. Clinical Outcomes of stereotactic MR Guided adaptive radiation therapy for high-risk lung tumors. Int J Radiat Oncol Biol Phys. 2020;107(2):270 -278.
- Finazzi, T., Palacios, M., Spoelstra, F., Haasbeek, C., Bruynzeel, A., Slotman, B., et al. Role of On-Table Plan Adaptation in MR Guided Ablative Radiation Therapy for Central Lung Tumors. Int J Radiat Oncol Biol Phys. 2019;104(4):933 -941.
