Demonstrating the value of MR-guidance
Target volume coverage and OAR sparing in an initial GenesisCare SABR patient cohort
Stereotactic ablative radiotherapy (SABR) has been widely adopted as the standard of care in the treatment of oligometastatic disease1,2. Whilst reported acute toxicity of SABR is generally mild, late toxicity can be significant. In the SABR-COMET trial the rate of grade 2-5 adverse events was 29% for the experimental arm versus 9% for the control arm (p = 0.03)1. This effect may be further augmented in abdominopelvic malignancies given the complex surrounding anatomy and proximity to multiple mobile organs at risk (OARs) in this location.
Stereotactic MR-guided online adaptive radiotherapy (SMART) with breath hold gating has the potential to improve therapeutic window when delivering high ablative doses near mobile OAR3,4 (Figure 1).
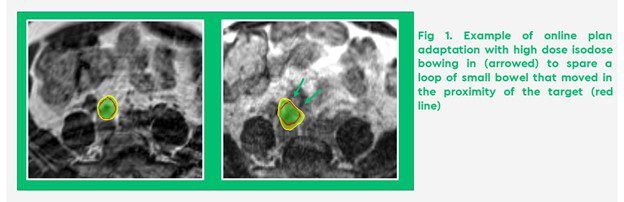
To quantify the benefit of daily adaptive SMART to abdominal and pelvic nodal metastases in our patient population, we carried out a planning study looking at target volume coverage and OAR dose, comparing baseline plans with non-adaptive and daily adaptive plans
All patients were treated on the 0.35 T MRIdian Linac (ViewRay Inc., Oakwood, OH, USA), with prescribed dose ranging from 30Gy to 50Gy in 5 fractions using daily adaptive treatments, using a previously published fully adaptive workflow.
Fifteen patients were included in the analysis resulting in 69 fully adaptive fractions.
- Without daily adaptation, 61% of fractions would have resulted in OAR constraint violation. This was reduced to 6% with daily plan adaptation.
- Plan adaptation resulted in a mean 27% reduction of the maximum point dose and dose delivered to 0.5 cc of duodenum and stomach within 3 cm of PTV
- Significant improvement in minimum GTV dose (80% prescribed dose as compared to 71% for non-optimised plans, p=0.02), volume of GTV receiving 95 and 100% prescribed dose (99% vs 97% and 98% vs 93%, respectively) and target PTV coverage (89% covered by 95% isodose vs 83%, p<0.0001) (Figure 2)
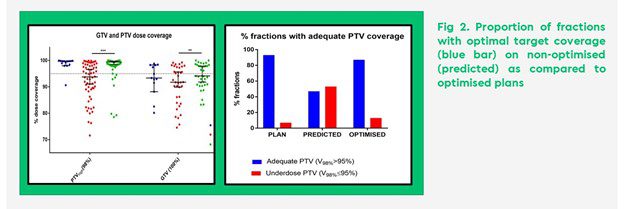
Our study, accepted for presentation at ESTRO 2022, highlights the benefits of MR guided daily online adaptation in the treatment of abdominopelvic lymph node metastases in terms of improved GTV and PTV coverage as well as OAR sparing.
Despite daily rigid image registration to GTV, we observed inter fractional variability in the position of the target as well as the surrounding stomach and duodenum. Inability to account for these differences using a conventional CT based linear accelerator might, in some instances, result in PTV coverage reduction even when using advanced respiratory motion management strategies.
Another important finding in our study was that SMART was effective at reducing the volume of stomach and duodenum receiving >30Gy with nearly 95% adaptive fractions meeting all OAR tolerance constraints. Conversely, in the absence of daily adaptation, OAR constraint violation was observed in 61% of treatment fractions.
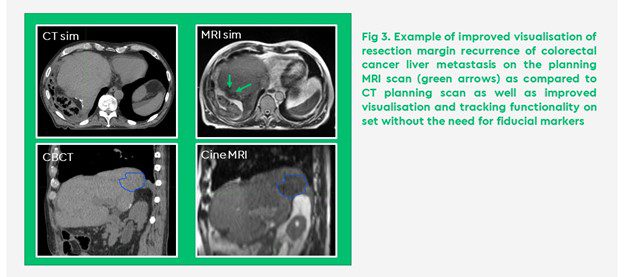
In addition, SMART utilises advanced image guidance with sufficient quality to visualise the tumour and OAR on treatment allowing for safe dose escalation in a completely non-invasive way and without the need for fiducial markers (Figure 3).
Our future work will include modelling of treatment data with the aim to expand SMART into other treatment sites. We are also committed to developing a prospective registry to monitor clinical outcomes and toxicity data with long term follow up to identify patients who stand to benefit most from this technology.
If you would like more information about how to access our MR Linac SABR service for your patients, please contact: sabr@genesiscare.co.uk
Kind regards,
Dr Kasia Owczarczyk, MD, MRCP, FRCR
Clinical Oncologist and MRIdian specialist