Radiation therapy (RT) kills cancer cells.3,4 It’s precisely targeted and intended to cause less damage to the surrounding healthy cells near the cancer.3,4 It can also be used to relieve pain and discomfort from cancer that has spread.3
In Australia it has been recognised that approximately 50% of cancer patients could benefit from radiation therapy.5 About 1 in 4 Australian patients will be treated with radiation therapy, during their cancer treatment journey.6
Radiation oncology as a specialist cancer treatment first started being used in Australia in the early 1900’s, with what would be regarded today as producing satisfactory results.7 In the past couple of decades technology has played an integral role in the evolution of the radiation therapy journey including simulation, planning, and treatment delivery, with improvements in the accuracy of delivery, quicker treatment durations, and efficiency.8
We are experts in cancer care and specialists in radiation oncology. We invest early in modern, evidence-based treatments and technologies so we can offer all of our patients a convenient and connected service experience. With 43 sites across Australia, the quality of our care is the same in regional communities as in metropolitan areas. Many of our doctors attend multi-disciplinary team meetings (MDTs) in their local area, discussing patient cases with specialists from different fields so most patients, particularly those presenting with complex conditions, can benefit from multi-disciplinary care.
Helpful additional Information
If you have any unanswered questions and would like further information, please head to the Targeting Cancer website for details about radiation therapy, or contact a member of the GenesisCare treatment team, via a centre near you.
There are two main types of radiation therapy techniques; external, where radiation is delivered from a machine and internal; where a medicine or even small steel “seeds” are inserted into the body to the cancer site.2,9 The most common form of internal radiation therapy is called brachytherapy.3
External beam radiation therapy represents the most common form of therapeutic radiation. It is delivered by a machine called a linear accelerator (linac), from the outside of the patient, and is usually delivered in intervals called ‘fractions’.2,3,9 This allows time between treatments for the healthy cells to repair and the cancer cells to die off.2
There are a number of different radiation therapy techniques used in Australia, developed to help treat different body sites.3 To learn more about some of the commonly used techniques we have created the below table aimed to help educate you about radiation therapy.
The type of radiation therapy technique recommended to treat cancer can vary based on factors including the type and grade of cancer, general health, age and individual preferences of each patient.3
The information provided in the below should not be used as a substitute for that recommended by a healthcare professional.
Treatment techniques comparison
Select the treatment technique below to read more.
First launched
First documented approximately a year after X-rays were discovered by Roentgen in 1896,10 with significant evolution occurring in last couple of decades.8
What is it?
EBRT uses high-energy radiation beams to destroy cancer cells.3
It is an umbrella term for all treatments with beams originating outside the patient, with many sub-types.3
EBRT can be given using different techniques and types of radiation.3
Target and Benefits
Aims to destroy cancer cells, to help prevent the spread of cancer, relieve symptoms, in combination with other treatments, adjuvant therapy, and in palliative care.3
How it works
It directs high-energy, targeted radiation beams at a tumour - delivered by a radiation machine called a linear accelerator (linac) that rotates around the patient during treatment to deliver radiation to the area with cancer from different directions.3
Most sessions last for 10-40 minutes, with the total session’s dependent upon type of cancer and prescribed dose to receive. It can’t be seen or felt.3
Common tumour stream examples
Localised cancers including: prostate, breast, lung, head and neck, and colorectal cancers.11
First launched
199412
What is it?
A type of EBRT, IMRT is a highly accurate type of conformal radiation therapy, able to be delivered by all modern linacs.3
Target and benefits
IMRT can deliver higher doses of radiation to tumours compared to conventional radiation therapy.13 It uses complex algorithms, while minimising damage to healthy tissue (e.g. salivary glands), which can mean fewer treatment toxicities are experienced by a patient.12
How it works
IMRT shapes and divides multiple beams of radiation into tiny beams (beamlets) to closely fit and match the tumour and its shape while healthy tissue nearby receives lower doses of radiation.3
Common tumour stream examples
IMRT can be used to treat tumours near critical structures, e.g. brain, head and neck.14
First launched
200715
What is it?
VMAT is a type of radiation therapy that maximises radiation to the tumour, whilst sparing healthy tissue to help reduce the risk of side-effects.15
A type of EBRT, VMAT is regarded as being a more advanced and efficient type of IMRT.15
Target & benefits
VMAT offers faster treatment times compared to other conventional radiation therapy types, at around 10 minutes.16
By precisely targeting the tumour, delivering a continuous beam of radiation therapy in an arc moving around the tumour, VMAT helps reduce radiation therapy dose to adjacent healthy organs, and this can mean less side-effects.3,15
How it works
It is a treatment customised to a patient’s anatomy, delivering small radiation beams to the tumour from a range of angles with the linac gantry rotating.17
Widefield VMAT is a technique that allows for large or curved surfaces to be treated, such as patients with skin cancer.16
Common tumour stream examples
A wide range of cancers including prostate, skin, head and neck, lung, gastrointestinal, and breast.16,17
First launched
1951 – 1953, with a number of advancements in treatment technology made during the past 50 years.18,19
What is it?
SRS is a non-surgical treatment for some types of cranial cancers.3,18
It was first developed to treat small targets in the brain that were not ideal for surgery, and it is a type of EBRT.3,18
Target & benefits
SRS aims to deliver high doses of targeted radiation therapy, which helps to reduce damage to nearby healthy tissue.18
SRS can treat multiple tumours, help lower the probability of local recurrence in some tumours, is very accurate so it can be tailored very precisely and can spare critical areas such as the brainstem.18
How it works
During treatment, the team deliver the radiation to the tumour with the help of imaging and careful planning. Patients are required to stay very still, and equipment is provided to help ensure this occurs.
SRS delivers highly focused beams of radiation from multiple angles to accurately target a tumour.
Depending on the type of SRS and tumour, the estimated treatment time is 30 minutes to 3 hours.18
Common tumour stream examples
Brain, secondary cancers that have spread to the brain, slow growing non-cancerous tumours.18
First launched
Mid 1990s20
What is it?
SBRT also known as SABR combines many small beams of radiation from different angles to target the exact shape of the tumour.3
SBRT is a specialised type of EBRT.
Originally derived from techniques and procedures of SRS.3,20
Target & benefits
SBRT aims to target tumours in the body with high doses of radiation therapy, while limiting the dose to surrounding healthy organs, and to lessen the chance of potential side-effects.3,21
The number of treatment sessions depends on individual patient circumstances.3
In some situations, SABR may be offered as an alternative to surgery for some types of tumours.22
How it works
SBRT uses modern technology aiming to very specifically target and help kill cancer cells.
CT scans are performed at the time of treatment, helping ensure treatment accuracy.3,22
SBRT was made possible by different technological advances including the ability to keep a patient quite still during treatment, tracking movement of the tumour, and more precise delivery of radiation therapy.22
Common tumour stream examples
A range of cancer types including lung, prostate, liver, bone/spine, and kidney cancers.22
First launched
1968, with modern developments and updates in 2006 and 2017.23,24
What is it?
Gamma Knife radiosurgery is a non-invasive stereotactic radiosurgery (SRS) technique for tumours, as well as other abnormalities that can develop in the brain.23
Target & benefits
Gamma knife radiosurgery is a form of radiation therapy technology which can deliver large doses of targeted radiation, thereby helping to minimise radiation to the surrounding healthy cells.23,25
Gamma knife radiosurgery uses computer-guided radiation to treat intracranial cancers.23
Gamma Knife helps to enable the treatment of hard-to-reach brain tumours that may not be easily treated with conventional surgery.26
How it works
Gamma Knife radiosurgery delivers multiple converging beams of gamma radiation toward a central point to deliver high dose radiation to a specific target within the brain and to avoid significant doses to normal brain tissue.23
Common tumour stream examples
A variety of brain disorders including, brain metastases, meningiomas, pituitary adenomas, acoustic neuromas, arterial venous malformations, trigeminal neuralgias.23
First launched
1990s, with some improvements in terms of treatment planning and technology that shapes radiation beams.27
What is it?
CyberKnife is a non-invasive robotic radiosurgery system that uses radiation to treat small tumours and some medical conditions.27
Target & benefits
The CyberKnife delivers high doses of radiation to the tumour (tracked real-time via fiducial markers), aiming to minimise exposure to nearby healthy tissue.27
The CyberKnife was originally developed to treat intracranial tumours.27
How it works
A robotic arm delivers radiation beams to the tumour.
Fiducial markers (gold seeds) are required to be inserted (via a separate procedure prior to radiation treatment) to more accurately track the tumour - not usually required for many other radiation therapy techniques.27
Treatment session duration estimated at 30-60 mins.27
Common tumour stream examples
Brain, intracranial, lung, kidney, and prostate cancers.27
First launched
Late 1990’s28
What is it?
ART is a type of treatment that continuously modifies the plan during treatment to account for any changes in a patient’s anatomy.29
Target & benefits
ART offers the ability to design personalised treatment plans for a patient based on anatomy and tumour type.29
Potential side-effects may be minimised due to the adaptive nature of the technique.
During treatment the team can monitor response in real-time and modify the treatment plan.29
How it works
Before each treatment session, a patient will be imaged (either CT or MRI scan) to capture the current anatomy of the tumour and surrounding organs.29
Once the imaging scan has been received, the plan will be adjusted to precisely target the tumour, at its position, and thereby to minimise damage to healthy surrounding tissues.29
ART is usually considered for tumours where anatomical changes may occur during treatment.
Common tumour stream examples
Tumour types may include head and neck, cervical, lung, prostate, bladder, and rectal.29
First launched
201429
What is it?
The MR-Linac technology, also known as Magnetic Resonance Image-Guided Radiotherapy (MRIgRT), combines high resolution magnetic resonance imaging (MRI) scanning offering improved soft-tissue visualisation, daily real-time imaging and the opportunity for adaptive radiation therapy to adjust for any anatomical changes that take place during treatment.30, 31
Target & benefits
Real-time magnetic resonance imaging allows the MR-Linac to show the exact position and shape of the tumour during treatment, so it can target the tumour more precisely.31
The treatment team can adapt the radiation plan on the day of treatment based upon tumour location, with real-time tumour tracking allowing for adjustments to the radiation beam.31
The MRI helps provide detailed superior soft tissue imaging (in comparison to some other radiation therapy techniques that use a CT scan), enabling targeting precision of the tumour.29
How it works
Patients who are recommended to be treated via MR-Linac will have a planning appointment, then treatment sessions, and follow-up appointment(s).
Treatment duration (depending on plan, tumour type), may take 45-60 minutes including MRI scan.
Before each treatment session, the patient will have a new MRI scan which will be compared with planning scans. Any movements of the tumour and internal organs will be considered, and the treatment plan will be carefully adjusted and adapted to account for these changes.
Tumours that are located close to other major organs where limiting damage to healthy tissue is important, or in organs that move a lot.
Common tumour stream examples
Tumour types may include lung, rectal, prostate, pancreatic, liver, kidney, and bladder.29
First launched
2001, with technological evolution since, including advanced 3D cameras and thermal imaging.32,33
What is it?
DIBH is a technique used to help treat cancer in the breast or chest wall.3,34
DIBH has evolved since 2001 to use advanced 3D cameras and thermal imaging to monitor the patient’s surface (SGRT, see below).32,33
Target & benefits
The aim of this technique is to minimise damage to the heart, which can occur due to the precise targeting used.3,34
DIBH is a breathing technique that involves a patient taking and holding a deep breath while radiation therapy is delivered. It is combined with special imaging equipment to monitor tiny movements of the patient’s body.33
How it works
Prior to treatment, patients have a planning session with a Radiation Therapist who will explain the DIBH process and take a CT scan.3
During each treatment session, the patient will take a deep breath and hold it while the radiation is delivered.35
By using specific monitoring equipment, the radiation is only delivered when the patient is still.33
Common tumour stream examples
DIBH aims to minimise radiation exposure to the heart and lungs and can be used when treating certain types of cancers located in the breast, chest, or abdominal area.3,34
First launched
2000s36
What is it?
IGRT uses X-rays and scans before, and during treatment.3
Target & benefits
IGRT can offer more precise treatment via imaging of the tumour and adjustments to a patient plan.37
It can help to reduce potential side-effects and help improve tumour control.37
IGRT is beneficial to treat tumours that are close to sensitive organs or those that move.37
How it works
IGRT uses imaging of the tumour before or during radiation treatment.
The images are compared to initial simulation images to design a treatment plan.37
Common tumour stream examples
IGRT can target cancers that move during, or between, treatment sessions such as lung, prostate, liver and pancreas.37
First launched
Early 2000’s38
What is it?
The SGRT technology uses modern 3D camera technology to help track and monitor patient movements during set-up and treatment and does not require tattoos to guide treatment delivery.39,40
Target & benefits
SGRT features accurate positioning and motion tracking that eliminates the need for permanent tattoos on the patient’s body.39,40
SGRT is often used to assist DIBH treatment for breast cancer to help minimise the radiation dose to the heart.39
Treatment sessions are generally faster than other radiation therapy techniques, as the set-up is quicker, and this means less treatment time.39
How it works
SGRT uses an infrared camera that provides the radiation therapist with thousands of reference points on your skin, which aims to precisely track any movements in real time and help support accurate delivery of radiation to the targeted area.39,40
Common tumour stream examples
Breast, brain, head and neck, lung, liver, pancreatic, prostate, and gastrointestinal cancers.39
First launched
1901 with recent improvements in imaging used.41
What is it?
Brachytherapy is a common type of internal radiation therapy, used to target and destroy cancer cells.3
Target & benefits
It is one way to give a high dose of radiation to a tumour, however, aims for a low dose to reach surrounding tissue and organs. This may mean fewer side-effects.42
How it works
Sealed radioactive sources are placed inside the body, close to or inside the cancer.42
The duration and type of brachytherapy recommended depends upon the type of cancer and dose.3
Dose rates can include, high-dose-rate (HDR), low-dose-rate (LDR).41,42
Common tumour stream examples
Breast, cervical, prostate, uterus, and vaginal cancers.42
Following the initial consultation with a radiation oncologist and treatment team, which usually includes a discussion about test results, health assessment, whether radiation therapy is suitable for treatment, plus possible side-effects and patient consent, a patient will undertake a couple of steps to prepare the treatment plan.
This process, managed by a Radiation Therapist, simulates the patient in the proposed treatment position with measurements taken via a CT-scan. These images are used to determine the location of the tumour and the surrounding organs.3
One technological advancement that has helped improve the simulation process is the ability to acquire images during the breathing cycle, allowing the capture of the inspiratory and expiratory phase. The combination of respiratory assessment and acquisition of imagery via CT is called 4D-CT. This helps the treatment team better understand tumour motion (during a patient’s treatment).3
Another technique used today is Deep Inspiration Breath Hold (DIBH), in which a patient’s scan is taken in a breath-hold position. While the patient is holding their breath, the CT-scan is taken and can mean that the final images are clearer. Treatment can then be delivered in the same breath-holding position, and this eliminates any uncertainty of tumour motion due to breathing.33
A procedure utilised for some types of radiation therapy is the insertion of fiducial markers inside the tumour, which takes place separately before simulation. Fiducial markers may be used during treatment delivery to better track the tumour.44
After the simulation appointment, the images acquired are sent to the Radiation Oncologist to assess and determine the dose, total treatment sessions, and to contour the tumour and surrounding tissue and organs (using specific computer software).
There have been some technological advances (in the computer software used) that mean additional images can be supplied via MRI and PET scans, which can be utilised with the CT scan, and these help to ensure the contours marked by the Radiation Oncologist are accurate.3
Further technological evolution has seen the culmination of advanced simulation, radiation planning software and upgrades to linear accelerator technology that allow delivery of a high ablative radiation dose called stereotactic body radiation therapy (SBRT) – also known as stereotactic ablative radiotherapy (SABR).3 The planning software can program the linear accelerator to deliver very high ablative doses to the tumour, with minimal dose to the surrounding tissues. Because these doses are very high, treatment can be delivered in only 1 to 5 fractions.3
Once approved, the treatment data is transferred to a Radiation Therapist to program the treatment, as detailed by the Radiation Oncologist ensuring that the tumour is treated, and surrounding tissue is spared.
On the day of treatment, the patient is set-up in the exact same position as the CT simulation appointment, and then they receive their radiation therapy treatment.
Depending on the radiation therapy technique being used, the treatment session may take between 10-40 minutes.3
The total treatment sessions depend upon the personalised treatment plan created for each patient. The remainder of the treatment sessions should be similar to the first session.3
One example of a technological advancement in treatment delivery, is the ability to image the tumour and/or surrounding soft tissue daily before treatment to verify the position by matching with the planning CT scan (at simulation). A treatment technique such as the MRIgRT/MR-Linac combines high resolution MRI scanning providing improved soft-tissue visualisation, daily real-time imaging and the opportunity for adaptive radiation therapy to adjust for any anatomical changes that take place during treatment, and to replan as needed.30,31
Types of imaging technology
In Stereoscopic X-ray imaging (the technology used for CyberKnife)27 only high-density objects are visible - such as bones and fiducial markers (for example, gold seed fiducial markers used in some prostate cancer treatments).
In Image A (below), you can see the gold seed fiducial markers appearing white.
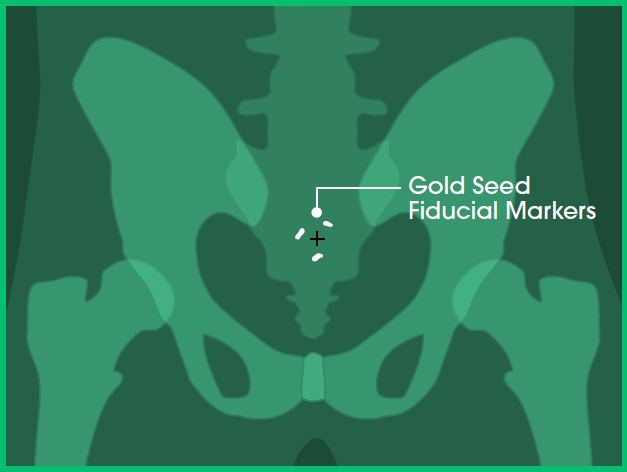
Image A: Front view
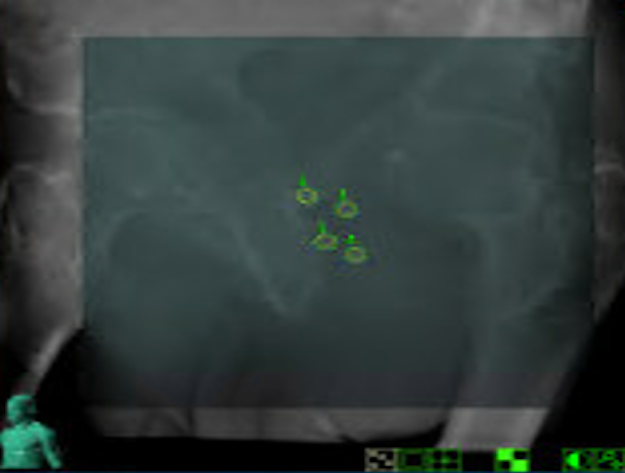
Image B: Clinical imaging scan
With Cone Beam Computed Tomography (CBCT), you can visualise a full 3D alignment of the patient and tumour with surrounding anatomy.
While high-density objects like bones and fiducial markers are still visible (as in stereoscopic X-ray), CBCT also allows you to view soft tissues in three dimensions.
In images A, B and C, you can see the:
- Prostate in red
- Bladder in blue
- Bowel in orange
- Fiducial markers in white
- Planned target volume outlined in yellow (where the radiation will hit)
In image D, which is an example of a CBCT, you can see the:
- Planned target volume outlined in blue
- Bowel outlined in orange
This level of imaging is standard at GenesisCare and is used in our Linac machines for Image-Guided Radiation Therapy (IGRT) and Adaptive Radiation Therapy (ART).
Note: Certain SABR treatments cannot be performed with stereoscopic imaging alone. Examples include targeting tumours or nodes in the chest, abdomen, or pelvis where critical healthy structures need to be visualised before delivering treatment that stereoscopic imaging cannot see.
Without knowing the tumour's position in relation to nearby healthy organs, there is a risk of overdosing healthy tissue, which may lead to side effects.
CBCT technology also enables real-time tumour tracking when combined with fiducials. It can capture images during treatment delivery, visualising fiducials within the tumour. This means treatment can be monitored in real time and paused if the tumour moves out of position.
This technology is standard for SABR treatment of prostate cancer.
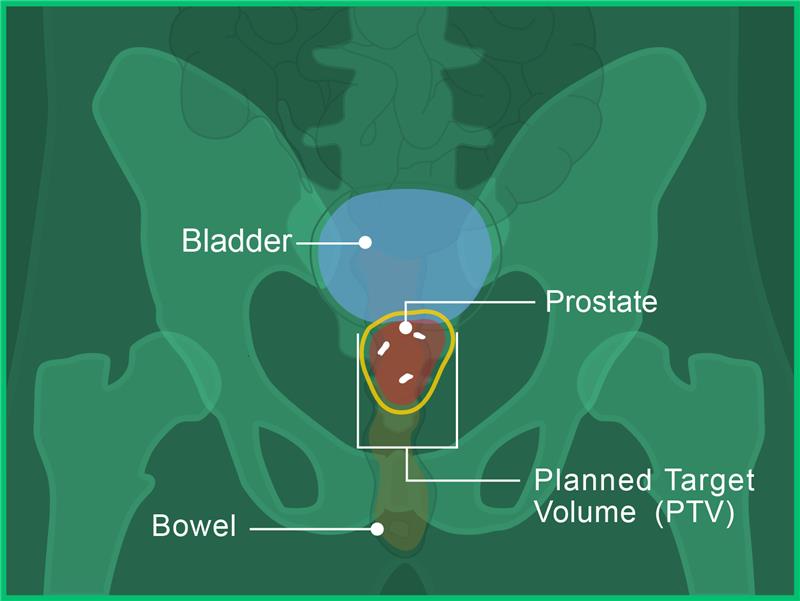
Image A: Front view
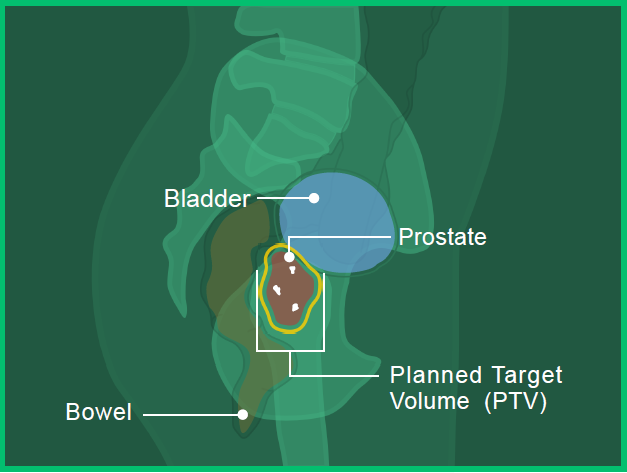
Image B: Side View
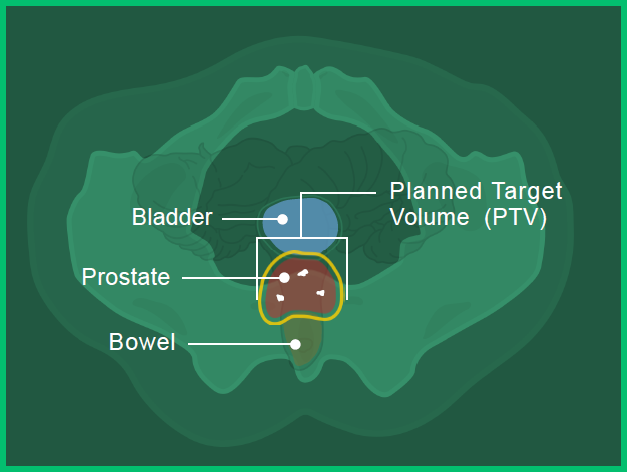
Image C: Bottom View
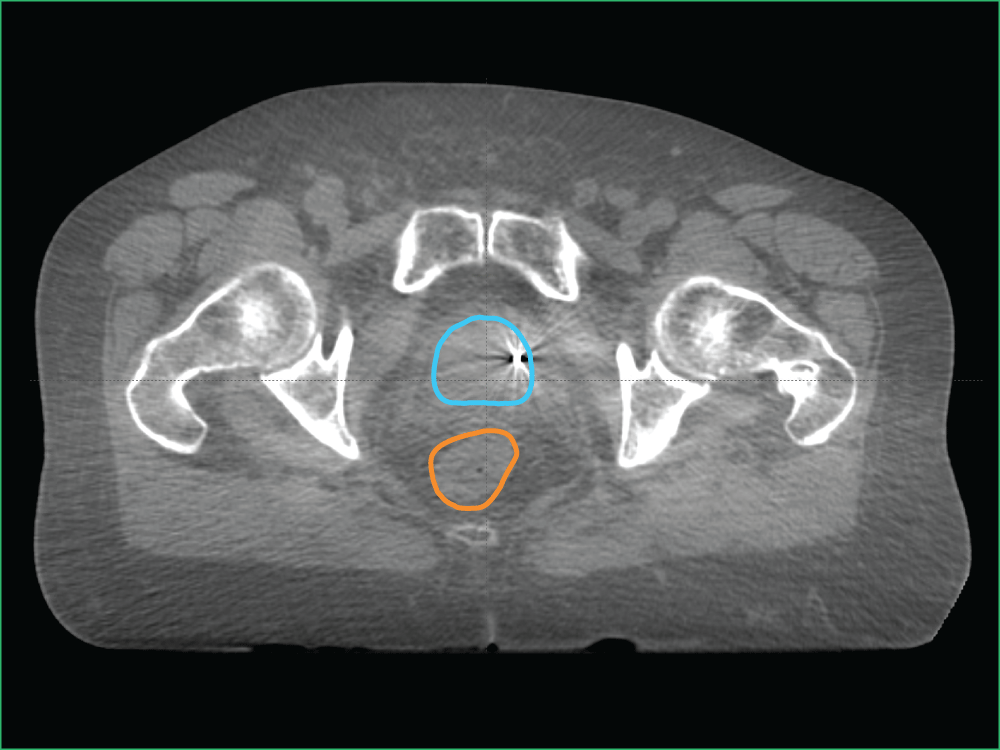
Image D: Clinical imaging scan
Magnetic Resonance Imaging (MRI) enables even more detailed visualisation of soft tissue, in addition to high-density objects.
In image A, you can see the:
- Prostate in red
- Bladder in blue
- Bowel in orange
- Fiducial marker in white
- Planned target volume outlined in yellow (where the radiation will hit)
Image B shows the high level of detail available with MRI-guided radiation therapy. You can see the:
- Planned target volume outlined in blue
- Bowel outlined in orange
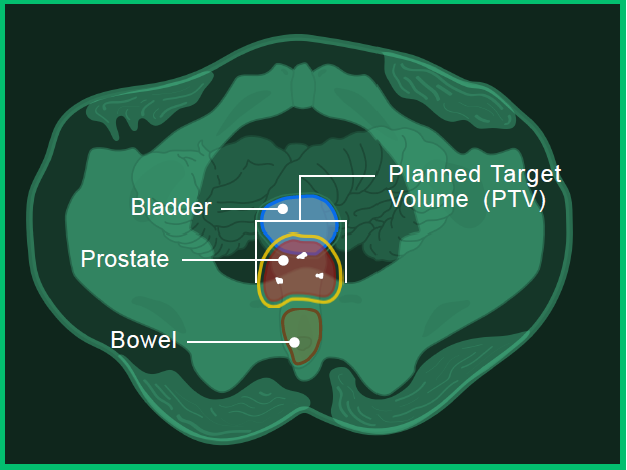
Image A: Bottom view
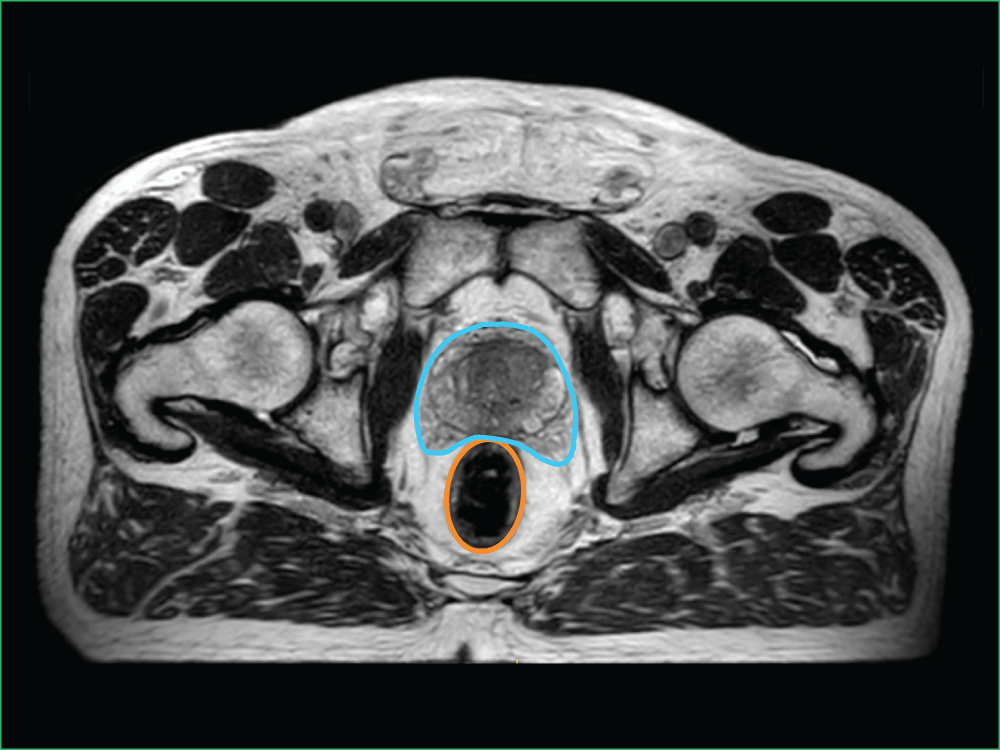
Image B: Clinical imaging scan
For any patient, it’s recommended to ask their Radiation Oncologist and treatment team any questions they have concerning treatment, including technique, planning and potential outcomes.
All cancer treatments may have side effects, yet the type and severity of side effects will vary between individuals.9 Patients can ask their treating doctor for detailed information about potential side effects which may be experienced with treatment recommended.
Recommendations for staying well during treatment:
- Get as much rest as possible44,45
- Aim for a wholefood, varied diet, and we also encourage thinking about eating foods that interest a patient rather than what they think they should eat44
- Appetite changes are common, and this may include taste changes or nausea. A suggestion to help manage this is to eat small, frequent snacks and avoid smells that can bring on a feeling of nausea.44
- Drink lots of water44
- Reach out to support groups and others who have had cancer treatment45
- Recording any side effects in a diary or journal46
- Speaking to the treating doctor about incorporating some gentle exercise into the weekly routine44,45
- It is important to acknowledge when feeling fatigued and to rest when needed44,45
- Cancer Council. Treatment. Available from: https://www.cancer.org.au/cancer-information/treatment (accessed March 2025.
- Majeed H, Gupta V. Adverse Effects of Radiation Therapy. [Updated 2023 Aug 14]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK563259/ (accessed March 2025).
- Cancer Council. Understanding Radiation Therapy. Available from: https://www.cancer.org.au (accessed March 2025).
- Walter and Miller's textbook of radiotherapy : radiation physics, therapy and oncology. Symonds, Paul (R. Paul); Walter, J. (Joseph). Edinburgh ; New York : Churchill Livingstone; 2012.
- Barton MB, et al. Radiother Oncol 2014 Jul;112(1):140–4.
- Gabriel G, et al. Radiother Oncol 2015 Nov;117(2):386–9.
- Morgan GW, Aust N Z J Surg 1998 Mar;68(3):225–35.
- Fiorino C, et al. Mol Oncol. 2020 Mar 19;14(7):1500–1513.
- Chaput C. Can Fam Physician. 2021; 67(10): 753–57.
- Do Huh H and Kim S, Prog Med Phys 2020;31(3):124–134.
- Cleveland Clinic. External Beam radiation Therapy (EBRT). Available from: https://my.clevelandclinic.org/health/treatments/24008-external-beam-radiation-therapy-ebrt (accessed March 2025).
- Cho B. Radiat Oncol J 2018;36(1):1–10.
- RadiologyInfo.org. Intensity-Modulated Radiation Therapy (IMRT). Available from: https://www.radiologyinfo.org/en/info/imrt (accessed March 2025).
- Cheung KY. Biomed Imaging Interv J 2006;2(1):e19.
- Teoh M, et al. Br J Radiol 2011;84(1007):967–996.
- Fogarty GB, et al. Biomed J Sci & Tech Res 2018;4(1):3719–3726.
- Cleveland Clinic. Volumetric Modulated Arc Therapy (VMAT). Available from: https://my.clevelandclinic.org/health/treatments/17626-volumetric-modulated-arc-therapy-vmat (accessed March 2025).
- Hynes PR, Das JM. Stereotactic Radiosurgery (SRS) and Stereotactic Body Radiotherapy (SBRT) [Updated 2023 Jul 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK542166/ (accessed March 2025).
- Solberg, T.D., Siddon, R.L., Kavanagh, B. (2012). Historical Development of Stereotactic Ablative Radiotherapy. In: Lo, S., Teh, B., Lu, J., Schefter, T. (eds) Stereotactic Body Radiation Therapy. Medical Radiology(). Springer, Berlin, Heidelberg.
- Timmerman RD, et al. J Clin Oncol 2014;32(26):2847–2854.
- Tsang Maverick WK. Journal of Thoracic Disease 2016;8:S517–S527.
- Radiation Oncology Targeting Cancer. Stereotactic Ablative Radiotherapy in AU & NZ. Available from: https://www.targetingcancer.com.au/radiation-therapy/stereotactic-ablative-radiotherapy-sabr/ (accessed March 2025).
- Desai R and Rich K. Mo Med 2020; 117(1):33–38.
- Ganz JC. (2022). Chapter 4: Gamma Knife evolving instrumentation. In: Ganz JC (ed) Progress in Brain Research. Elsevier. Vol268, Iss 1, pp 49–63.
- Farris JC, et al. Head Neck. 2022;44(11):2571–2578.
- Tomoyuki K et al. Neurol Med Chir (Tokyo) 2010;50:737–48.
- Kurup G. J Med Phys 2010;35(2):62–64.
- Brock KK. Semin Radiat Oncol 2019;29(2):181–184.
- Hall WA, et al. CA Cancer J Clin 2022;72:34–56.
- Henke, LE et al. Clin Oncol (R Coll Radiol). 2018;30(11):720-27
- Castelluccia A, et al. Int J Environ Res Public Health 2022;19:10800.
- Sixel KE, et al. Int J Radiat Oncol Biol Phys 2001;49(1):199–204.
- Latty D, et al. J Med Radiat Sci. 2015;62(1):74–81.
- Stowe HB, et al. Breast Cancer (Dove Med Press). 2022;14:175–186.
- Bergom, C. et al. Front Oncol. 2018;8:87.
- Herrmann H, et al. Radiologe 2019;59(Suppl 1):21–27.
- RadiologyInfo.org. Image-guided Radiation Therapy (IGRT) Available from: https://www.radiologyinfo.org/en/info/igrt (accessed March 2025).
- Mast M and Perryck S. Tech Innov Patient Support Radiat Oncol. 2022;22:37–38.
- Freislederer P, et al. Radiat Oncol 2020;15(1):187.
- Freislederer P, et al. Radiother Oncol 2022;173:188–196.
- Mayer C, Gasalberti DP, Kumar A. Brachytherapy. [Updated 2023 Jun 15]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK562190/ (accessed March 2025).
- Chargari, C et al. CA Cancer J Clin. 2019;69(5):386–401.
- RadiologyInfor.org. Fiducial Marker Placement. Available from: https://www.radiologyinfo.org/en/info/fiducial-marker#:~:text=Fiducial%20markers%20are%20small%20metal,tumor%20to%20help%20guide%20treatment (accessed March 2025).
- Cancer Council Australia [website]. Nutrition for People Living with Cancer. Last updated July 2022. Available from: https://www.cancer.org.au/cancer-information/downloadable-resources (accessed March 2025).
- Cancer Council Australia [website]. Exercise for people living with cancer. Last updated August 2023. Available from: https://www.cancer.org.au/cancer-information/downloadable-resources (accessed March 2025).
- Cancer Council Australia [website]. Understanding Chemotherapy. Last updated August 2024. Available from: https://www.cancer.org.au/cancer-information/downloadable-resources (accessed March 2025).
- Cancer Council Australia [website]. Cancer, work and you. Last updated June 2023. Available from: https://www.cancer.org.au/cancer-information/downloadable-resources (accessed March 2025).

You are leaving our website
You are now leaving our website. GenesisCare do not control this content and therefore are not responsible for its accuracy or reliability.



