Theranostics
Theranostics enquiry
Contact our theranostics team directly to find out how we can help you.
Theranostics is a developing new approach to cancer treatment which may be suitable for some patients who have not responded to other therapies or who have complex metastases (e.g. where cancer has spread to multiple sites).
Based on current research and clinical experiences, it is most likely to be suitable for patients with metastatic prostate cancer and neuroendocrine tumours.1,2 Your GenesisCare oncologist will determine if theranostics may be suitable for you.
How it works
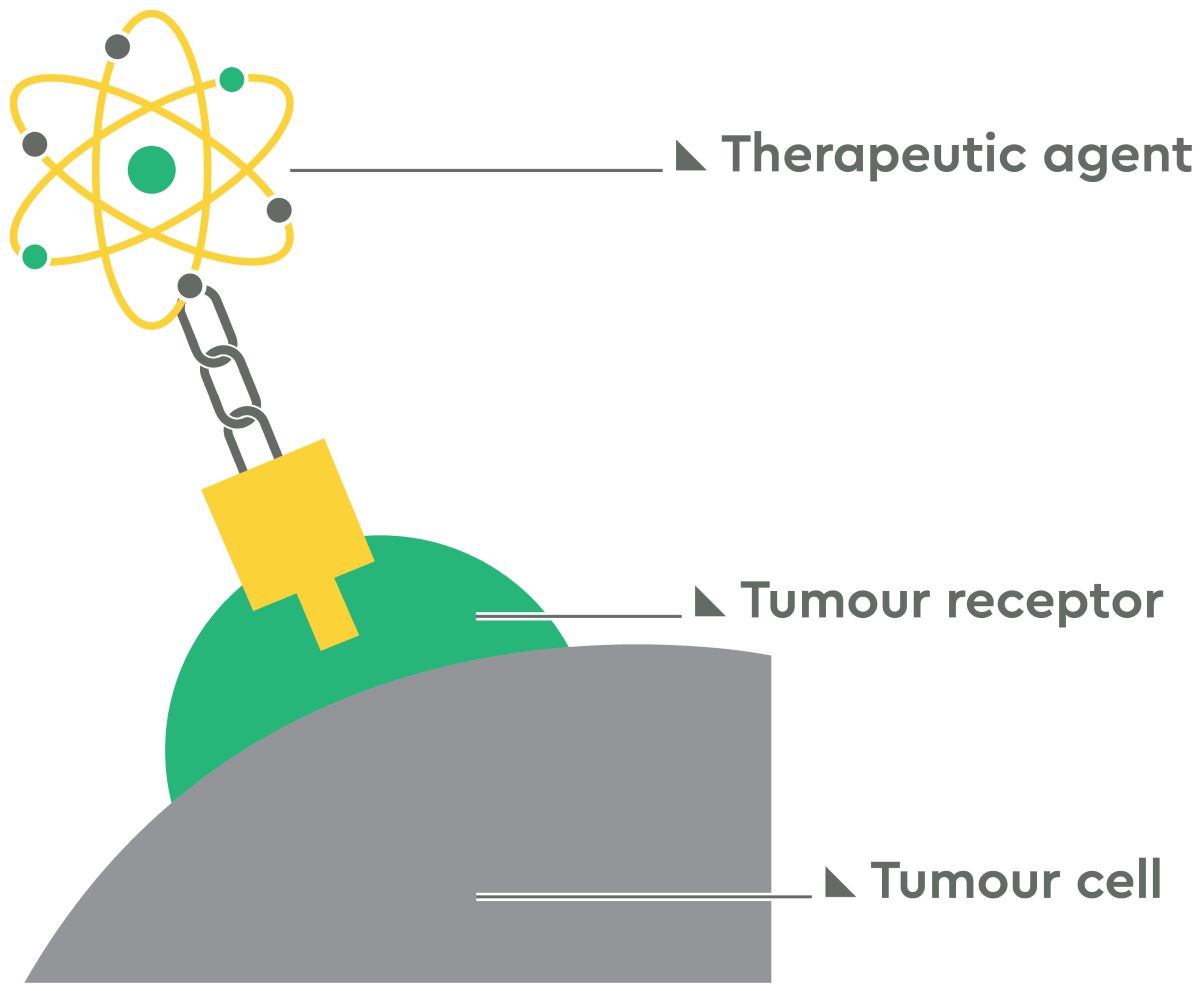
Theranostics is a personalised approach to treating cancer, using both diagnosis and therapy tools as part of the treatment. Theranostics uses PET scan imaging (a special type of scan) to see if specific targets, known as tumour receptors, are present on tumour cells.
If these targets are present and visible on the scan, a radioactive drug is used to treat the tumours. The drug is given as an injection and selectively targets the tumour cells while avoiding healthy areas.1,2 Most of the radioactive drug that doesn’t reach the target is quickly passed out of the body.
Theranostics treatment may involve one or more therapeutic goods which have not been reviewed for safety, quality, or efficacy by the Therapeutic Goods Administration (TGA). Some patients may be eligible to access this treatment under the Special Access Scheme. The cost of treatment may be an out-of-pocket expense.3
Theranostics patient journey
It is important that you feel informed and supported on your Theranostics treatment journey. We are here to answer any questions or concerns you may have.
Here are areas people commonly ask about:
Before treatment commences, your Nuclear Medicine Physician will explain the treatment process, treatment options and any potential side effects. You will receive a care plan tailored to your needs.
Any out of pocket costs associated with your treatment will be discussed with you in a clear and simple way before you commence treatment.
In order to develop your treatment plan, you will be required to attend a few appointments. These will include a visit to an imaging department and pathology centre for your blood tests.
Prior to receiving your cycle of treatment, your Nuclear Medicine Physician will review your results and confirm your treatment plan.
A nurse will assess your overall health status.
You will be given a tour of the department and an overview of what to expect.
A nuclear medicine technologist or nuclear medicine physician will deliver your treatment via an injection and monitor you regularly. A nurse will check that you are hydrated throughout the day by ensuring you have enough liquids or may put you on a drip if necessary. The treatment will last approximately 4 hours.
Where is theranostics offered?
We currently have four centres in Australia offering theranostics treatment for suitable patients.
Theranostics enquiry
Contact our theranostics team directly to find out how we can help you.
Our partnerships
For enquiries regarding partnership opportunities, please contact us via theranosticsreception@genesiscare.com
Our theranostic specialists

Dr Aviral Singh
MD PGDipCard MSc FAfNM FRACP - Clinical Head of Theranostics and Nuclear Medicine
Nuclear Medicine Physician


- Gomes Marin JF, Nunes RF, Coutinho AM, et al. Theranostics in Nuclear Medicine- Emerging and Re-emerging Integrated Imaging and Therapies in the Era of Precision Oncology. Radiographics. 2020;40(6)-1715-1740.
- Vu TM, Loveday BPT, Behrenbruch C, Hollande F, Heriot AG. Theranostics- a fifth pillar of contemporary cancer care? ANZ J Surg. 2022;92(11)-2782-2783.
- Therapeutic Goods Administration. Special Access Scheme: Guidance for health practitioners accessing unapproved therapeutic goods. V1.0 January 2023.

You are leaving our website
You are now leaving our website. GenesisCare do not control this content and therefore are not responsible for its accuracy or reliability.



