- Healthcare Professionals
- Oncology
- DCISionRT

Empowering women with breast cancer - introducing DCISionRT®
Biological profiling for personalised treatment of DCIS
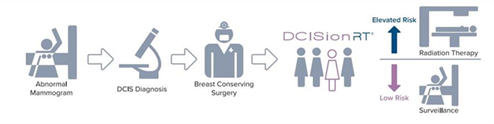
A diagnosis of Ductal Carcinoma in Situ (DCIS) can be confusing for patients. DCIS is the presence of abnormal cells inside a milk duct in one or both breasts. While DCIS itself is not life threatening, having DCIS can increase the risk of developing invasive breast cancer later in life. Most women who are diagnosed with DCIS will be recommended treatment. A typical treatment program would involve breast conserving surgery followed by radiation therapy.
Now there is a test available that predicts if radiation therapy will be beneficial. DCISionRT is a precision medicine test that provides information about a patient’s biological risk profile, empowering them to make a more informed decision about their treatment.
Patients can disuss the cost of this test during thier initial consultation with their Radiation Oncologist.
What is DCISionRT®?
DCISionRT®- the only precision medicine test to predict radiation therapy benefit for women with DCIS 1
DCISionRT® is a test that looks at the likelihood of a patient’s Ductal Carcinoma in Situ (DCIS) recurring after surgery, the risk of the disease spreading, and the impact of radiation therapy in reducing that risk.
DCISionRT assists patients and their doctor to select treatment based on the biology of the tumour, in addition to the traditional clinical and pathology factors.
DCISionRT- predicts radiation therapy benefit
DCISionRT helps doctors to formulate a personalised treatment plan to balance treatment benefit with the risk of side effects by assessing a patient’s underlying DCIS biology.
- Assesses the risk of DCIS coming back (recurring) and/or spreading
- Predict whether radiation therapy will benefit a patient
- The treatment is based on the biology of the tumour
- If the test is accessed through Genesis Care there is no additional cost - If no radiation therapy is needed at the time of results, there will be no cost to patients for the test.
- There are no additional procedures required to complete the test – DCISionRT is done using the existing tissue from the biopsy or breast surgery
DCIS is the presence of abnormal cells inside a milk duct in one or both breasts. DCIS is considered the earliest form of breast cancer and is sometimes referred to as Stage 0 (zero). DCIS is non-invasive, meaning it has not spread out from the milk duct, and has a lower risk of progressing to invasive breast cancer.
In itself, DCIS isn’t life threatening; however, having DCIS can increase the risk of developing invasive breast cancer later in life. Most women who are diagnosed with DCIS will be recommended treatment. This is because it is not possible to predict which women may go on to develop invasive breast cancer.
DCISionRT looks at women diagnosed with DCIS and assesses their 10-year risk of recurrence or of developing invasive disease with or without radiation therapy. It is the only test developed specifically for this purpose.1
The outcomes of the test show whether a patient’s risk is ‘low’ or ‘elevated’ and assesses the potential benefits of radiation therapy. The test results are then used as a decision tool for patients and their doctor when considering treatment options.
DCISionRT tests DCIS with 7 biomarkers, their interactions and their relationship to 4 factors about patients:
- Age at diagnosis
- How the lesion was discovered (by mammography or physical exam)
- The extent of the lesion (size)
- Whether the rim of tissue surrounding the lesion was free of DCIS (margin)
The more information patients and doctors have about an individual’s biological make-up, the more personalised they can make treatments for that patient. The additional biological information provided by DCISionRT may help to give insights into the risks a patient has of DCIS recurring and the likelihood of radiation therapy being an effective treatment option for a patient. A truly personalised approach.
Access to DCISionRT testing
Doctors can arrange the DCISionRT test for patients with DCIS. The test can be applied to the breast tissue sample taken as part of a biopsy or breast surgery; no additional procedure is required.
The DCISionRT test was launched in Australia recently, and whilst not currently on the Medicare Benefits Scheme or listed on the Therapeutic Goods Association in Australia, it is frequently utilised in the USA where many insurers fund the DCISionRT test. The patient’s tissue sample is sent offshore to California in the USA, to a PreludeDx CLIA-approved lab for analysis. GenesisCare is proud to be partnering with PreludeDx to develop the AUS-PREDICT registry to evaluate the effectiveness of the DCISionRT test on treatment decisions in patients, post-surgery.
The DCISionRT test has been validated in published studies with over 3,500 DCIS patients worldwide.1-4 DCISionRT is supported by Level 1b clinical evidence1
If you a doctor and patient with DCIS agree the test is appropriate, a tissue sample can be sent to PreludeDx in the United States to conduct the test.
The results will be shared with the doctor and a radiation oncologist within approximately three weeks, and a follow-up appointment may then be arranged with the doctor to discuss results and what those may mean for a patient’s treatment plan.
Patients can disuss the cost of this test during thier initial consultation with their Radiation Oncologist.
Get in touch
For more information contact your local centre or call or email us using the details below.
Email: DCISIONRT@genesiscare.com
References
Disclaimer
This information is provided for the sole purpose of disseminating educational information in relation to DCIS and DCISionRT. The content is not intended to provide medical advice or treatment recommendations and should not be relied on as such. The information does not take the place of professional or medical advice. While GenesisCare makes every effort to ensure the accuracy of the content, GenesisCare does not guarantee its accuracy, currency or completeness and does not accept any responsibility or liability for any injury, loss or damage incurred by use of or reliance on the content. Before relying on the content, you should carefully evaluate it and use your own professional discretion when determining its appropriateness for assessing the clinical needs of your patients (including considering any risks relevant to individual patients in the context of their full treatment protocol). The content includes information from third parties. This information is provided for convenience only and does not necessarily reflect the views of GenesisCare or signify that GenesisCare endorses such information.
Disclaimer:
This website is provided for information purposes only. Nothing on this website is intended to be used as medical advice, or to diagnose, treat, cure or prevent any disease. It should not be used as a substitute for your own health professional's advice. Any medical procedure or treatment carries risks. Before proceeding with treatment, you should discuss the risks and benefits of the treatment with an appropriately qualified health practitioner. Individual treatment outcomes and experiences will vary.