Adapting to a new way of treating
(Published in the BIR Blog)
Stereotactic ablative radiotherapy (SABR) is growing in importance in the curative cancer pathway. Increasingly, it offers patients the opportunity to enjoy relatively long periods of disease control where previously they would have been considered for palliative treatments. During COVID-19, the scales have tipped even further in favour of hypofractionated techniques because protocols have been revised to limit the risk of patient infection. More recently, attention has turned to stereotactic ablative MR-guided adaptive radiotherapy (SMART) – the most exciting development in radiotherapy for years, with the potential to treat previously inaccessible targets.
GenesisCare has been the first in the UK to adopt SMART, installing the first ViewRay MRIdian MR-linac just over a year ago. Since then, we have treated over 170 patients, some of which are the most challenging in the world from a radiotherapy perspective, such as pancreatic, central lung and now renal cell carcinomas. MRIdian sits within our SABR offering, which is run by a specialist team of oncologists, physicists, dosimetrists, and radiographers. Over an intensive 18 months, we have adopted a completely new way of working and overcome the challenges of a pandemic to treat patients not just from across the UK, but also from around the world.
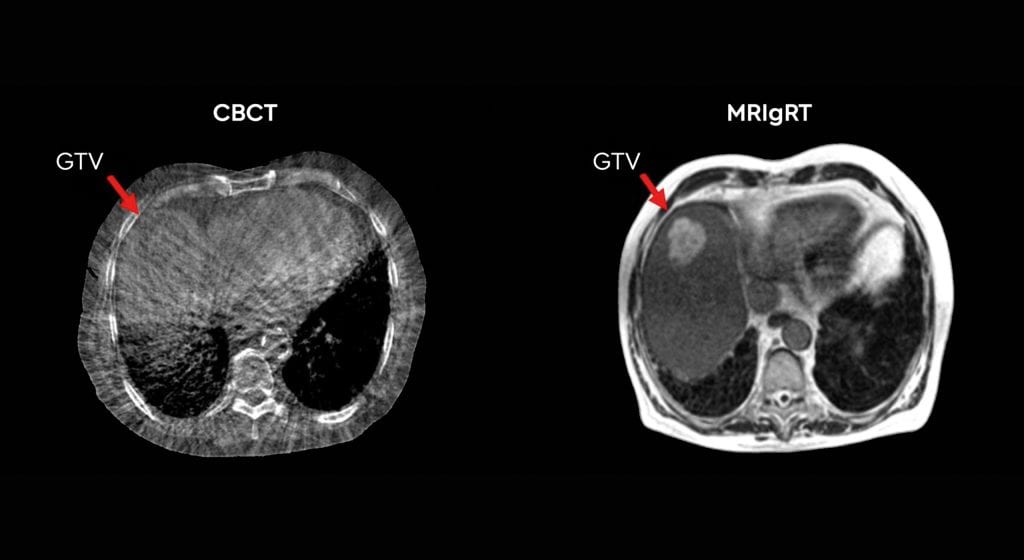
The MRIdian MR-linac combines a 0.35 T split superconducting magnet with a 6 MV linear accelerator. This gives it unique advantages over conventional external beam radiotherapy linear accelerators, which rely on kV cone-beam CT (CBCT) imaging, and enables an entirely new approach to treatment.
First, using MRI instead of CBCT provides superior soft-tissue visualisation. This increased imaging capability allows the treatment to be adapted at each fraction based on the daily position of the target and nearby organs at risk (OARs). This is in marked contrast to external beam treatment with CBCT, where anatomy captured in the CBCT is simply rigidly matched against a planning CT. This rigid registration is then used to calculate the movements required to shift the patient into the correct position for treatment.
Second, the MRIdian takes images continuously throughout the treatment period to not only monitor the patient position, but also turn the treatment beam on and off. This is carried out as the patient’s anatomy moves through the breathing cycle.
This combination of enhanced visualisation and real-time imaging adds a layer of certainty in the delivery of treatment.
- The MRIdian on-table adaptive planning system generates a new, optimised treatment plan for each fraction. This accounts for these day-to-day anatomical variations when the patient is in the treatment position.
- Treatment delivery is then automatically gated so that the dose is only delivered when the target is in the optimal position. The machine is able to monitor every intrafraction motion caused by breathing or organ-filling.
As a result of these factors, we can design plans which deliver a higher dose, more precisely than with conventional SABR. There is no need for invasive fiducial marker insertion and any uncertainty is removed. Moreover, we can reduce planning target volumes, remove internal target volumes, and minimise the amount of tissue irradiated.
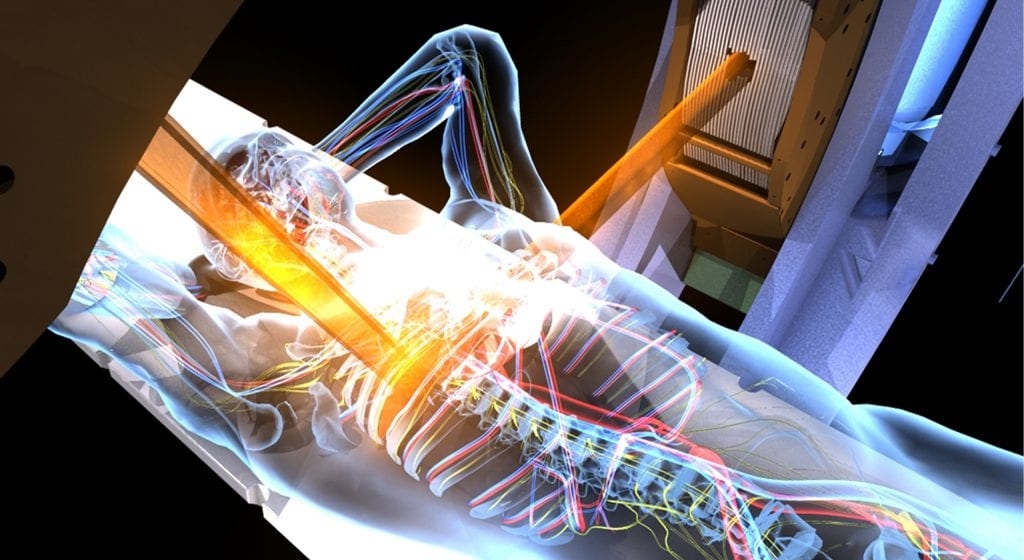
SMART has led to a paradigm shift in how some cancers are treated. In particular, it can benefit cancers in areas where there is significant inter- or intrafraction motion of either the target or OARs. Across the global community, MR-linac centres are now treating novel indications, such as renal, central lung and hepatobiliary tumours, and achieving clinical outcomes not previously thought possible. It is not simply a case of improving on an existing treatment – for some tumour types, SMART is facilitating new referral patterns for patients who may not typically be eligible for radiotherapy.
Pancreatic cancer is one such example and of all the tumour sites we are now treating at GenesisCare, this is undoubtedly the one that is breaking most ground, offering new hope for both clinicians and patients.
For decades, surgical resection and adjuvant chemotherapy and radiotherapy have been the cornerstones of primary and secondary hepatobiliary tumours and pancreatic cancer treatment. However, options are limited for many patients. Less than 20% are resectable at diagnosis and not all patients are fit enough for an operation or effective chemotherapy regimens. There is, however, emerging evidence of a dose-response relationship, proving that escalated radiation doses are associated with improved local control as well as overall survival in borderline resectable (BRPC) or locally advanced pancreatic cancer (LAPC). Conventional radiotherapy delivers a comparatively homogenous radiation dose to the target volume. In contrast, SABR treatments combine advanced image guidance systems, accurate dose delivery and hypofractionated regimes. This is to facilitate a deliberate heterogeneous dose distribution across the target. This means the radiation tolerances of surrounding OARs are respected, while the tumour receives a higher, ablative radiation dose. A number of SABR studies have yielded good results in the treatment of large hepatobiliary tumours, with 1-year local control exceeding 90% and acceptable toxicity. Furthermore, delivering these hypofractionated ablative doses of radiation over a shorter treatment schedule has the potential to reduce the burden of treatment on patients.
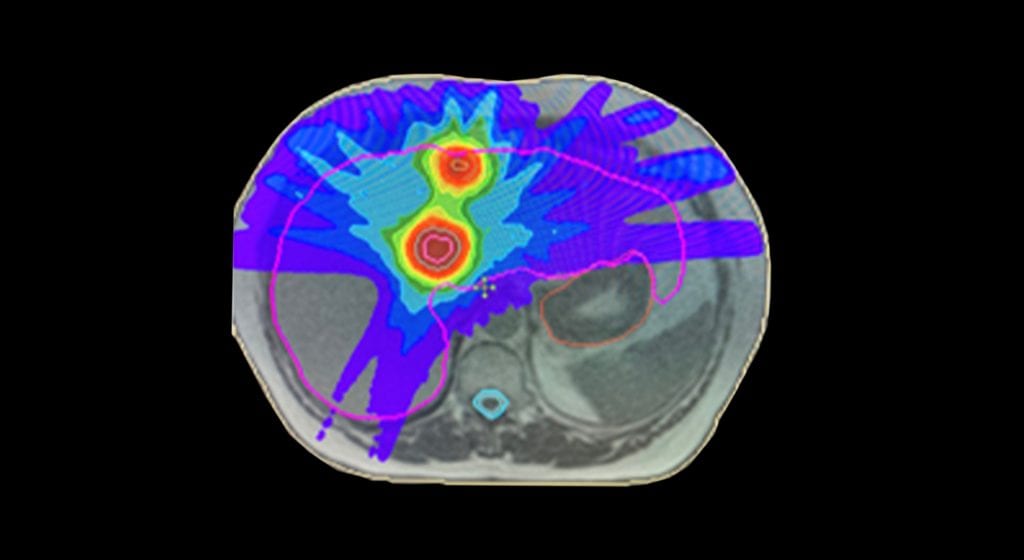
However, with conventional SABR this therapeutic approach is often limited by concerns regarding organ motion and the possibility of developing small bowel radiation toxicity. As a result, many patients are only being treated with systemic agents. This is a prime example of where the elements of SMART on an MR-linac can facilitate an effective radiation dose escalation, while still respecting the radiation tolerance of normal tissues and surrounding OARs. In fact, using an MR-linac, it has been possible to successfully increase the prescribed dose in patients with primary pancreatic cancer. The previous standard dose was 33 Gy in five fractions, but SMART enables us to escalate the prescribed up to 40 Gy or even 50 Gy in five fractions. At the time of writing, 30 patients have been treated on the MR-linac for pancreatic tumours at GenesisCare.
The significance of MR-linac as an innovation in cancer treatment can’t be understated and, although at GenesisCare we are offering it in a private setting, we are committed to sharing the benefits of this technology with the wider medical community. Patients with localised pancreatic cancer have variable access to precision radiotherapy in the UK. The n-SARS-CoV-2 pandemic has further disadvantaged this patient group by reducing the availability and safety of surgery and chemotherapy. Considering this, since 2020 GenesisCare in association with GenesisCare Foundation, UK charity, Pancreatic Cancer Research Fund, ViewRay and University of Oxford have been treating NHS patients with localised pancreatic cancer with SMART at no cost. The programme, which is run through a partnership with the University of Oxford, is generating preliminary clinical and patient-reported outcome data on a UK cohort. This will inform the design of subsequent randomised clinical trials and help to embed SMART in UK oncology practice.
With any new technology, there comes a learning curve. MR-linac represents a significant change in working practices. It demands a style of inter-disciplinary working which challenges the norms.
In a standard radiotherapy workflow, a patient will receive a treatment planning CT one to two weeks before the start of treatment. During this time, several steps are carried out by a team of dosimetrists, physicists, doctors and radiographers to produce a treatment plan ready for the patient’s first fraction. These steps include contouring the treatment target and OARs and optimising the machine parameters to deliver the prescribed dose to the target while sparing critical structures. This is followed by reviewing the dose distribution, checking the planning process to ensure no errors have occurred and performing an independent dose calculation.
As part of the on-table adaptive workflow, the time taken for this process must be reduced from days to minutes. In order to achieve this, close inter-disciplinary working between the team is required. The need to undertake a number of complex tasks during each adaptive treatment also increases the time for each fraction to around one hour.
The MRIdian workflow involves a Clinical Oncologist on-site during treatment to oversee the daily adaption. To maintain a treatment schedule at GenesisCare, this has meant that clinicians had to be trained to contour all areas of anatomy, often working outside their main area of specialism. Equally challenging was the need to acquire skills in MRI interpretation, which for some specialities is not routinely used as a diagnostic modality. These were all skills that needed to be honed and validated before any patients could be treated on the MR-linac. In our case, we spent many hours learning with colleagues in MR-linac centres of excellence around the world. Twelve months later, we are experts in this field and have treated over 170 patients.
There is a growing body of data as the global MR-linac community treats ever more and complex cases. We brought this international best practice to GenesisCare and have treated complex and challenging cases, including central lung, pancreas and reirradiation within our first year. We have many case studies available on our website genesiscare.com/mridian/case-studies. We already knew that the technology could deliver, but it was the confidence in our processes and the ability of our team to implement an adaptive workflow in a time-pressured environment, with a patient on the treatment table, which allowed us to embrace the opportunity that MR-linac presents in radiotherapy.
GenesisCare will install the second MR-linac in the UK in 2021. Through our MagNET programme, we are joining with NHS organisations to support education in the use of MR-guided radiotherapy. Enquiries to: James.Good@genesiscare.co.uk
Dr Ben George, Lead Physicist – MR Linac, GenesisCare