Conventional Linac primary prostate SABR
5 fraction prostate SABR now available on conventional Linac
Mr X is a 71 year old gentleman who presented with a raised PSA of 7.6 on a routine health check. His health was otherwise excellent and he had only very mild lower urinary tract symptoms (IPSS 2). He was referred to Urology who felt his prostate to be abnormal on digital rectal examination. A subsequent multiparametric MRI demonstrated a focus of restricted diffusion and early enhancement in the left mid gland, suspicious for malignancy and assigned as PIRADS-4. The PSA density was elevated at 0.25ng/m2.
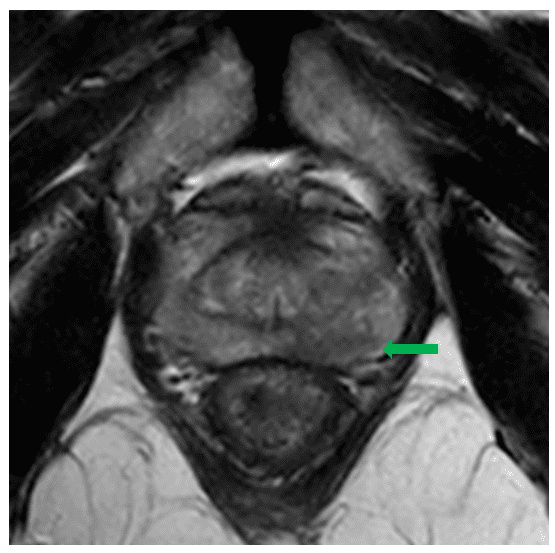
He went on to have a transperineal template biopsy under local anaesthetic at GenesisCare, Cambridge. This revealed adenocarcinoma of up to Gleason 4+4=8 in the left prostate core biopsies, with some cribriform pattern 4 and possible intraductal carcinoma. He underwent PSMA PETCT staging which excluded any distant metastases. His final stage was rT2aN0M0.
His case was discussed by the local Urology multidisciplinary team who recommended radical treatment with radiotherapy or surgery. Following dual consultations with his Clinical Oncologist and Urological Surgeon, Mr X opted for radiotherapy. This decision was influenced by the fact that he had a family member who had undergone adjuvant breast radiotherapy at another GenesisCare UK centre and had an excellent experience.
Since he expressed an interest in receiving a short course of treatment, his eligibility for primary prostate SABR was investigated.
The GenesisCare eligibility criteria for conventional Linac primary prostate SABR were achieved:
- Biopsy proven adenocarcinoma of the prostate, Gleason ≥ 6
- T-stage: T1c – T3b
- No evidence of lymph node or distant metastases
- Prostate volume ≤ 90 cc
- IPSS ≤ 19
- No TURP within 8 weeks
He also met none of the exclusion criteria:
- Previous irradiation in the pelvic region
- Medical issues likely to make androgen deprivation therapy inadvisable
- Prior active treatment for prostate cancer
- Life expectancy < 5 years
Subsequently his case was submitted for consideration by the Urology SABR Advisory Team (USAT), who agreed that he would be an appropriate candidate for conventional Linac primary prostate SABR. A mentor from the USAT was assigned to support his local treating Oncologist, who made contact quickly and offered to answer any questions. They arranged a training presentation at a mutually convenient time, which lasted approximately 30 minutes.
Following rectal spacer hydrogel insertion, the patient underwent a standard planning CT and MRI scan. The VOIs were drawn by his Oncologist and then approved by the USAT mentor without delay.
SABR planning images
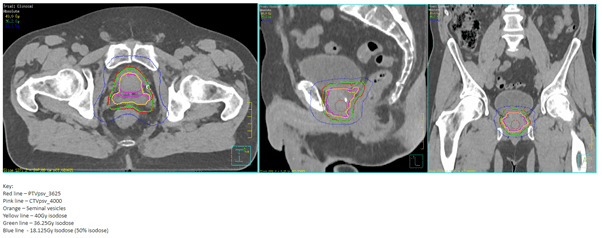
The patient received a dose of 36.25Gy in 5 fractions over 2 weeks. At the end of treatment he developed transient grade 2 acute radiation proctitis, which responded to steroids suppositories and resolved completely by 6 weeks post-treatment. His follow up PSA at 6 months is undetectable, although to date he remains on adjuvant androgen deprivation therapy. He has no symptoms of late pelvic radiation toxicity.
If you would like more information about how to access these treatments for your patients, please don’t hesitate to contact sabr@genesiscare.co.uk
Kind regards,
Dr Jenny Nobes
Consultant Clinical Oncologist
GenesisCare UK