Simple SABR for Oligometastatic disease
“Using SABR within the GenesisCare network was a sensible, quick process with excellent support from the SAT team. It allowed the patient to be treated with state-of-the-art radiotherapy at the closest centre to her home.”
The role of stereotactic ablative radiotherapy (SABR) as a single therapy or part of a combined approach in the treatment of metastatic cancer is steadily evolving.
Data from studies such as the SABR COMET phase II trial and the NHS England Commissioning Through Evaluation scheme has shown very encouraging results. In patients with extracranial oligometastatic disease the use of SABR was associated with both an increase in survival but also low toxicity.
In the UK GenesisCare provides both ‘simple’ and ‘complex’ SABR treatments. Simple SABR means non-spine bone, peripheral lung, and pelvic lymph node metastases. It is available at all UK GenesisCare centres.
Complex SABR means spine, liver, pancreas, central lung, abdominal nodes and reirradiation and is available at centres where local expertise is available, and demand requires it. In addition, SABR is also available using the MR Linac with daily adaptation in both Oxford and the Cromwell Hospital for challenging anatomical locations.
All consultants who have attended the GenesisCare Credentialing Programme, or who can demonstrate relevant experience in the NHS can refer and treat simple SABR cases. To date approximately half the cases treated across the UK GenesisCare network have been ‘simple’.
My patient, Mrs B was diagnosed with metastatic carcinoma of the lung in 2018 when she presented with shortness of breath and cough. After investigation she was found to have a T3N2M1a adenocarcinoma of the right lung with bilateral pulmonary nodules and a single rib metastasis. She was commenced on Carboplatin, Pemetrexed and Pembrolizumab in September 2018 but needed to stop after 3 cycles due to immune-related hepatitis and nephritis.
Although imaging in February 2019 suggested a response, she unfortunately progressed in July 2019 and was challenged with single agent Pembrolizumab. She has subsequently remained on this treatment with a good radiological response. Towards the end of 2019 she did receive a short palliative course of radiotherapy with 20Gy in 5 fractions to the right hilar mass for symptom control.
This was tolerated well with good symptomatic effect.
In June 2021 Mrs B remained well however a PET/CT scan showed a growing, avid left upper lobe lesion (SUV 9.7) but no signs of progression elsewhere.

Figure 1 : PETCT scan showed the growing, avid left upper lobe lesion (SUV 9.7)
The images were reviewed in the local lung MDM and the peripheral left upper lobe nodule was felt to represent oligoprogressive disease. For this reason, I considered her potentially eligible for simple SABR to the lung nodule to maximise her disease control. On discussion she was keen to go ahead with treatment.

I completed a SABR Advisory Team (SAT) referral form and submitted it to the SABR MDT coordinator (SABR@genesiscare.co.uk). Her case was subsequently promptly discussed by the SAT team and approved for treatment. The radiotherapy referral was then completed on Horizon and she was quickly scheduled for her pre-treatment and radiotherapy appointments. The overall process was very straightforward and supported throughout by the SABR and Maidstone Clinical teams.
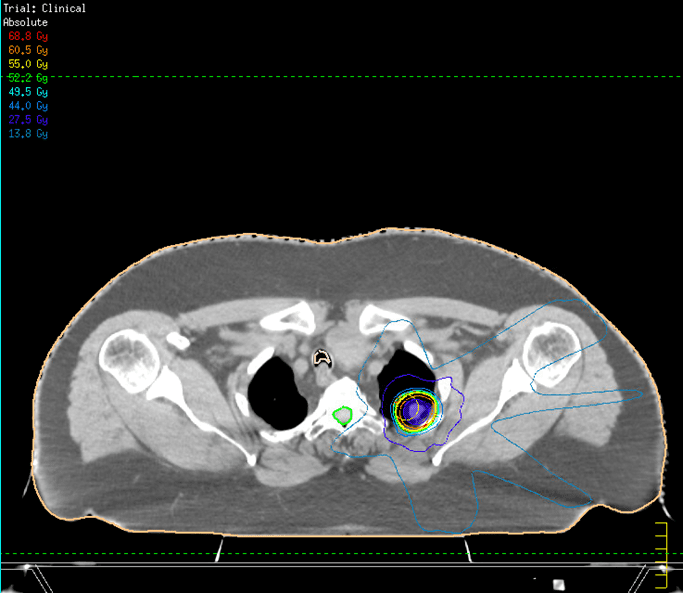
The peripherally located left upper lobe lung lesion was anatomically distant from the previous right-sided palliative radiotherapy field and was planned to receive 55Gy in 5 fractions over 2 weeks. The plan achieved all the mandatory OAR constraints.
Figure 2: Axial CT image of SABR plan with isodoses
The treatment was safely delivered, and Mrs B did not experience any significant acute side effects. She will be reviewed in clinic at 4 weeks with a plan for further imaging at 12 weeks to assess response.
If you would like more information about how to access these treatments for your patients, please don’t hesitate to contact sabr@genesiscare.co.uk
Kind regards,
Dr Tim Sevitt
Clinical Oncologist and MRIdian Specialist
GenesisCare UK