MRI-guided adaptive radiotherapy: Cutting-edge evidence across renal, prostate, and pancreatic cancer
Introduction
MRI-guided radiotherapy has emerged as a major technological advancement that directly addresses limitations of CT-based image guidance, including challenges in soft-tissue visualisation, organ motion, and proximity of many tumours to radiosensitive healthy tissues.
By integrating high-resolution, real-time MRI with radiotherapy delivery, stereotactic MRI-guided adaptive radiotherapy (SMART) enables clinicians to:
- visualise the tumour and surrounding organs during every treatment
- make daily anatomical adjustments through online adaptive planning
- use automated gating to control for motion
This approach allows for smaller margins, more precise targeting, improved healthy tissue sparing, and greater confidence in delivering ablative doses even in areas previously considered too high-risk.
As clinical experience grows, prospective trial data is beginning to define where MRI guidance adds measurable benefit. The following sections summarise emerging evidence from four studies across renal cell carcinoma, prostate cancer, and pancreatic cancer, illustrating how MRI-guided SABR may expand treatment options across multiple disease sites.
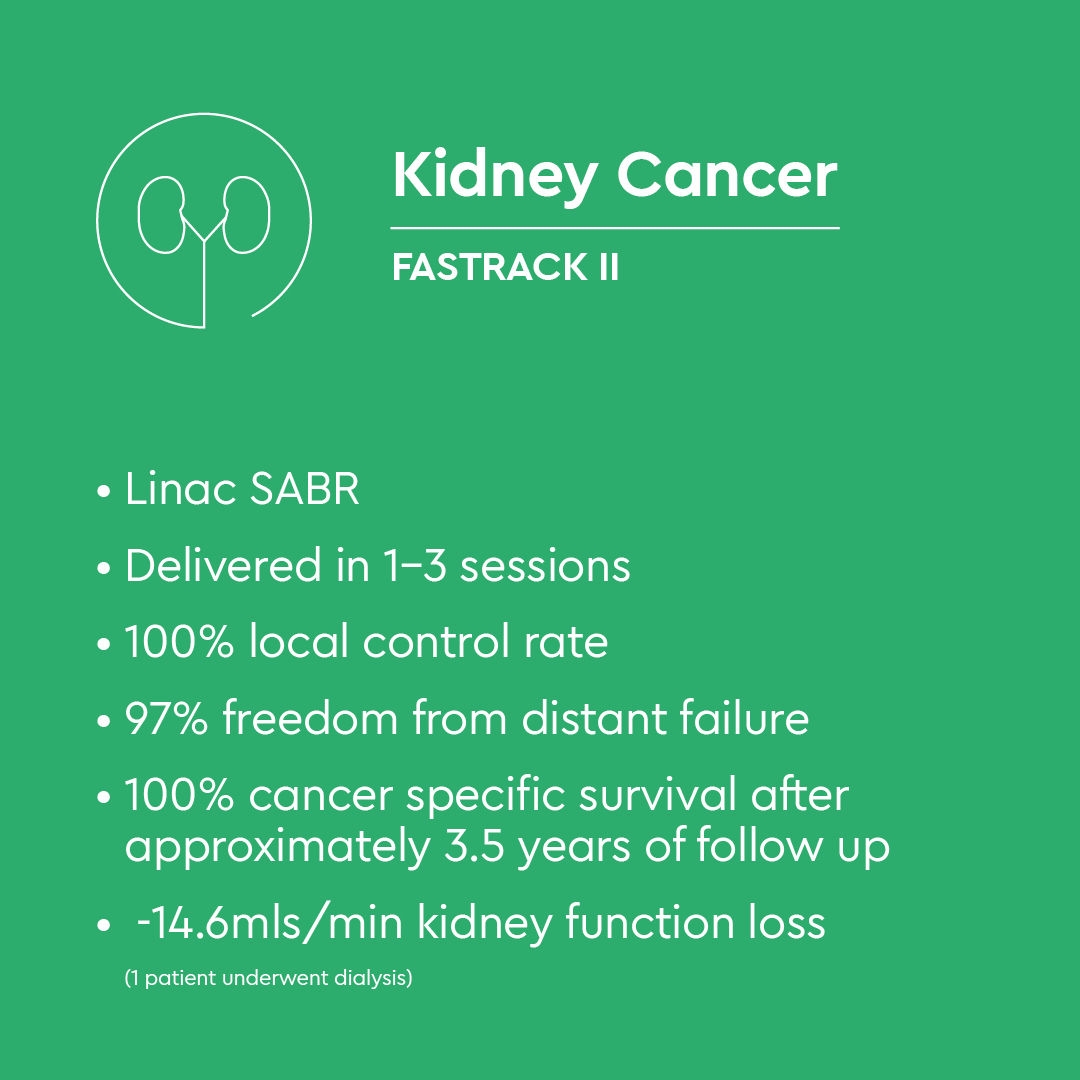
FASTRACK II - Phase II evidence in renal cell carcinoma
Study design and patient population
The FASTRACK II trial (NCT02613819) was designed as the first international, multicentre Phase II study to evaluate the efficacy and safety of SABR as definitive therapy for primary renal cell carcinoma.(1)
FASTRACK II was a non-randomised Phase II study conducted across eight centres in Australia and the Netherlands. Seventy patients who had a biopsy-confirmed diagnosis of primary renal cell carcinoma, and were considered medically inoperable, high risk for surgery, or had declined surgical management-initiated treatment between July 2016 and February 2020. Median age was 77 years, and median tumour size was 4.6 cm.
Treatment consisted of either 26 Gy in one fraction for tumours 4 cm or smaller or 42 Gy in three fractions for tumours measuring more than 4 cm up to 10 cm.
Key outcomes
- Local control at 12 months was 100% (p < 0.0001), meeting the primary endpoint and exceeding the predefined threshold for clinical significance
- No local failures or cancer-related deaths were observed during follow-up
- Grade 3 toxicity occurred in 10% of patients
- No grade 4 adverse events were reported
- Renal function after SABR remained acceptable
- The majority of treated patients (70%) had documented tumour growth on prior surveillance imaging, demonstrating that SABR provided effective disease control even in actively progressing tumours
Interpretation
FASTRACK II provides the first multicentre prospective evidence supporting SABR as a safe and effective non-surgical curative treatment for primary renal cell carcinoma. In a predominantly T1b or larger cohort, SABR achieved 100% local control with no cancer-related deaths and an acceptable toxicity profile. These findings justify progression to a future randomised controlled trial comparing SABR directly with surgery and support the integration of SABR into treatment pathways for medically inoperable renal tumours.
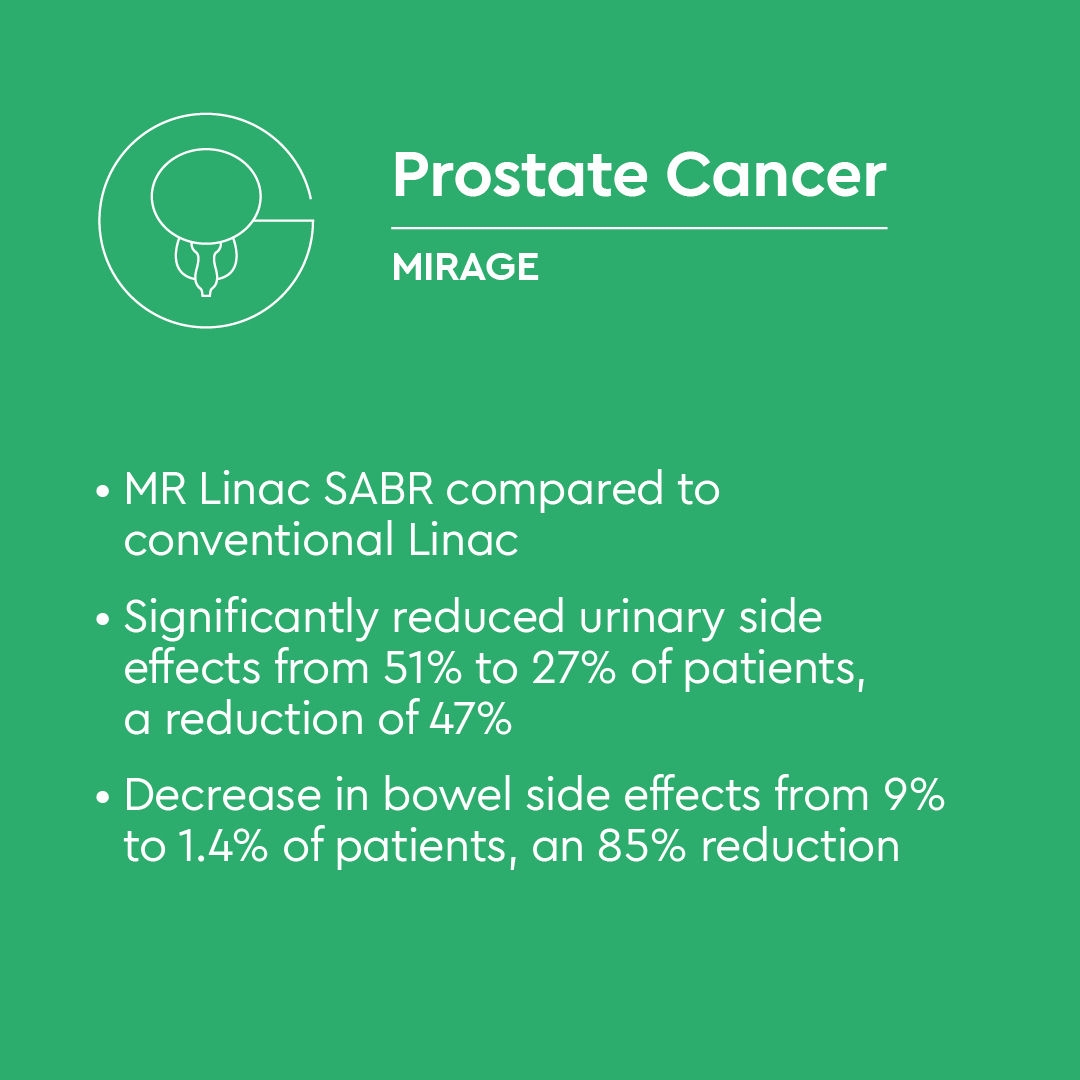
MIRAGE Trial - Phase III evidence in prostate cancer
Study design and patient population
The MIRAGE trial (NCT04384770) was designed as the first Phase III randomised study to compare the acute toxicity of MRI-guided versus CT-guided stereotactic radiotherapy. (2)
Between May 2020 and October 2021, 156 men with localised prostate cancer were randomised 1:1 to receive 40 Gy in five fractions using either CT guidance with a 4 mm planning margin or MRI guidance with a 2 mm margin. Treatment was delivered on either conventional Linacs (CT arm) or an 0.35T MR-Linac (MRI arm). MRI guidance did not involve daily adaptive planning. Rectal spacers and elective nodal irradiation were permitted at physician discretion.
Key outcomes
- Acute grade ≥2 genitourinary toxicity occurred in 43.4% of CT-guided patients and 24.4% of MRI-guided patients (p = 0.01)
- Acute grade ≥2 gastrointestinal toxicity occurred in 10.5% of CT-guided patients and 0% of MRI-guided patients (p = 0.003)
- A clinically significant deterioration in IPSS at one month occurred in 19.4% of CT-guided patients compared with 6.8% of MRI-guided patients (p = 0.01)
- A clinically meaningful decline in EPIC-26 bowel scores occurred in 50% of CT-guided patients compared with 25% in the MRI arm (p = 0.001)
- No unexpected safety signals were reported
Interpretation
The MIRAGE trial provides high-quality evidence that MRI-guided prostate stereotactic radiotherapy significantly reduces acute urinary and bowel toxicity compared with CT guidance. Importantly, these benefits were achieved without online adaptive planning, suggesting even greater potential for toxicity reduction when MRI guidance is combined with daily adaptation.
EMERALD Trial - Phase I evidence in pancreatic cancer
Study design and patient population
The EMERALD Phase I trial (ISRCTN10557832) evaluated whether SMART could safely deliver ablative radiation doses using daily adaptive planning and real-time motion management.(3)
This single-centre Phase I study enrolled 25 patients with localised or locally recurrent pancreatic cancer between August 2022 and October 2023. Patients received one of the following regimens: 50 Gy in five fractions, 39 Gy in three fractions, or 25 Gy in a single fraction. All treatments were delivered with daily online adaptive planning and real-time tumour tracking on a 0.35 T MR-Linac. Thirty-seven total fractions were analysed dosimetrically.
The EMERALD trial was funded by GenesisCare and delivered through our collaboration with the University of Oxford
Key outcomes
- Planning target volume coverage (PTVHigh V95%) was maintained at 98% or higher across all dose levels
- All gastrointestinal organ-at-risk constraints, including those for the stomach, duodenum, and bowel, were met for every fraction delivered
- Re-optimised plans consistently improved target coverage compared with non-adaptive baseline and predicted plans
- No dose-limiting toxicities or safety-related interruptions were observed.
Interpretation
The EMERALD trial confirms that SMART is technically feasible for pancreatic cancer and that ultra-hypofractionated ablative schedules can be delivered safely using daily adaptive planning. These results support ongoing research into single-fraction and extreme hypofractionation strategies for pancreatic cancer.
SMART Trial - Phase II evidence in pancreatic cancer
Study design and patient population
This Phase II trial (NCT03621644) evaluated whether ablative SMART (50 Gy in five fractions) could deliver high biologically effective doses safely after induction chemotherapy.(4)
This prospective, multicentre, single-arm trial enrolled 136 patients, including 56.6% with locally advanced disease and 43.4% with borderline resectable disease. All patients had received at least three months of prior chemotherapy, had no evidence of distant progression, and had a CA19-9 level of 500 U/mL or lower. SMART was delivered on a 0.35 T MR-Linac. Elective coverage was permitted, and both surgery and systemic therapy were allowed after treatment. Median follow-up was 22.9 months from diagnosis and 14.2 months from SMART.
Key outcomes
- Two-year overall survival from diagnosis was 53.6%, and two-year overall survival from SMART was 40.5%
- Surgical resection was achieved in 34.6% of patients following SMART
- Late grade ≥3 gastrointestinal toxicity definitely related to SMART occurred in 0% of patients
- Late grade ≥3 toxicity probably related to SMART occurred in 4.6% of patients
- Late grade ≥3 toxicity possibly related to SMART occurred in 11.5% of patients
- Acute grade ≥3 SMART-related toxicity was not observed
Interpretation
The Phase II study demonstrates that SMART provides favourable overall survival and low rates of severe late gastrointestinal toxicity in borderline resectable and locally advanced pancreatic cancer. The ability to achieve surgical resection in more than one-third of patients suggests that SMART may play an important role in facilitating downstaging.
Clinical implications of MRI-guided SABR
The collective evidence from renal cell carcinoma, prostate cancer, and pancreatic cancer highlights several important developments in MRI-guided and stereotactic radiotherapy:
- Real-time MRI guidance enhances the accuracy of treatment delivery in mobile and anatomically complex tumour sites
- SMART allows for safe dose escalation, particularly in the pancreas, where conventional SABR is limited by gastrointestinal toxicity
- MRI-guidance reduces acute toxicity in prostate cancer, improving patient tolerance during extreme hypofractionation
- SABR provides a viable curative option for medically inoperable renal cell carcinoma, with long-term tumour control rates approaching those of surgery
- Daily adaptive planning expands the feasibility of ablative treatment in settings where conventional radiotherapy has historically been constrained
MRI-guided SABR at GenesisCare
GenesisCare was the first healthcare provider in the UK to introduce the MR Linac technology, reflecting our commitment to delivering the most advanced radiotherapy technologies for patients. The MR Linac integrates a high-definition MRI scanner with a linear accelerator, enabling true MRI-guided stereotactic ablative radiotherapy (MRIgRT), offering capabilities far beyond conventional image-guided radiotherapy.
Unlike CT-based systems, which rely on intermittent imaging and may require larger margins to account for uncertainty, the MR Linac acquires continuous high-quality MR images during treatment. This provides unparalleled soft-tissue definition and allows clinicians to observe anatomical motion as it happens, without exposing patients to additional ionising radiation.
A unique feature of the MR Linac is its ability to track the tumour in real time and automatically gate the beam if the target moves outside the treatment boundaries. This ensures that radiation is delivered only when the tumour is precisely in position, allowing for tighter margins, greater sparing of normal tissue, and improved confidence when treating mobile or anatomically complex tumours.
Daily online adaptive planning is a core advantage of the MR Linac workflow. Clinicians can re-contour the tumour and surrounding organs at risk for each fraction, adjust the plan accordingly, and account for day-to-day variations in bowel gas, stomach filling, tumour position, or patient anatomy. This enables safe delivery of ablative doses in areas where conventional radiotherapy may be limited by toxicity risks.
The MR Linac is available at our GenesisCare centres in Oxford, London, and Surrey, providing access to world-class MRI-guided adaptive radiotherapy for patients throughout the UK. Our dedicated teams of clinical oncologists, physicists, and radiographers have extensive experience delivering MRI-guided SABR and continue to contribute to national and international research in this rapidly evolving field.
Dr James Good
Clinical Director for SABR
GenesisCare UK
Refer today
To refer to one of our specialists or to find out how we can help you and your patients, contact us at the below email.
References
1. Siva S, Bressel M, Sidhom M, et al. Stereotactic ablative body radiotherapy for primary kidney cancer (TROG 15.03 FASTRACK II): a non-randomised phase 2 trial. Lancet Oncol. 2024;25(3):308-316. doi:10.1016/S1470-2045(24)00020-2
2. Kishan AU, Ma TM, Lamb JM, et al. Magnetic Resonance Imaging-Guided vs Computed Tomography-Guided Stereotactic Body Radiotherapy for Prostate Cancer: The MIRAGE Randomized Clinical Trial. JAMA Oncol. 2023;9(3):365-373. doi:10.1001/jamaoncol.2022.6558
3. George B, Moreno-Olmedo E, Teoh S, et al. Dosimetric outcomes of ultra-hypofractionated adaptive MR-guided SABR for pancreatic cancer: EMERALD Phase 1 trial [Poster presentation]. ESTRO 2025 Annual Meeting (2025)
4. Parikh PJ, Lee P, Low DA, et al. A Multi-Institutional Phase 2 Trial of Ablative 5-Fraction Stereotactic Magnetic Resonance-Guided On-Table Adaptive Radiation Therapy for Borderline Resectable and Locally Advanced Pancreatic Cancer. Int J Radiat Oncol Biol Phys. 2023;117(4):799-808. doi:10.1016/j.ijrobp.2023.05.023