- Healthcare Professionals
- Oncology
- Treatments
- Radiotherapy
- MRIdian guided SABR for cervical cancer
MR-guided SABR boost for cervical cancer
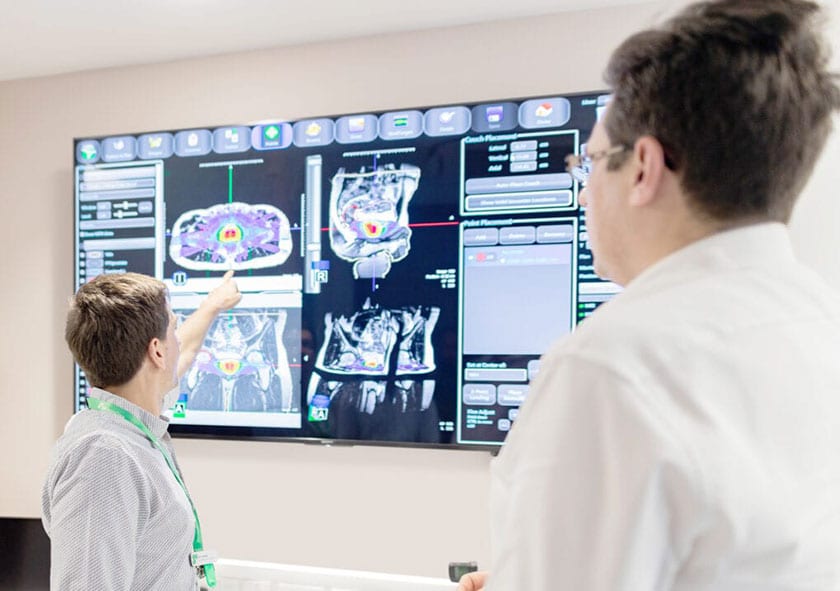
Stereotactic ablative body radiotherapy (SABR) delivered with MRI guidance (MRgRT) offers patients with locally advanced cervical cancer the most advanced form of external beam radiotherapy treatment available today.
At GenesisCare, we deliver MR-guided SABR on the MR Linac. This state-of-the-art technology combines a linear accelerator with an MRI scanner, offering high-definition visualisation together with real-time, on-table adaptive radiotherapy, bringing a new level of confidence to SABR delivery.
We’re proud to be the first independent healthcare provider to introduce MR-guided radiotherapy to patients in the UK. We’re committed to being part of global research efforts into applications of MR-guided radiotherapy, helping provide access to more patients and offer treatment options to a wider range of conditions, including cervical cancer.
The current standard of care for locally advanced or medically inoperable cervical cancer is radical pelvic chemoradiotherapy.1 This is typically a 5-week course of external beam radiotherapy plus weekly cisplatin chemotherapy, followed by a high dose rate (HDR) brachytherapy boost.
An alternative to brachytherapy
Brachytherapy is not always possible or suitable, due to medical or technical reasons or patient refusal. In these cases, an external beam boost using IMRT (intensity modulated radiotherapy) or VMAT (volumetric modulated arc therapy) is usually considered. However, the typical dose of 16-20 Gy in 8-11# is not usually curative. There is also evidence of lower survival in patients who are treated with an external beam boost13.
MR-guided SABR boost provides an alternative treatment option for patients where brachytherapy is not feasible. The MR Linac offers improved soft tissue visualisation, enabling protection for organs at risk (OAR, notably bladder, rectum and bowel) as well as improved targeting, leading to fewer side effects and improved dose to the target.
At GenesisCare, providing the option of a SABR boost delivered on the MR Linac means we can now offer a high-precision, non-invasive treatment option in 5# over five alternate days to patients with cervical cancer unable to receive brachytherapy. This could lead to improved target coverage and therefore improved survival outcomes.
How to refer a patient
Our referrals process is designed to be straightforward and rapid.
Patients deemed suitable for MR-guided SABR will be referred from a Gynae MDT to one of our MRIdian-credentialled clinical oncologists. All cases will be discussed in the GenesisCare SABR MDT before a decision is made.
The minimum data set required for referrals is:
- Cervical radiotherapy consent form
- MR-Linac supplementary consent form
- Booking form (electronic/paper)
- Referral letter (referring the patient to the clinical oncologist
- Histology report
- Staging pelvis MRI, CT, CAP and PET-CT
- Week 5 diagnostic MRI (to assess response prior to SABR boost)
- GenesisCare MRIdian assessment pro forma
This treatment is available to patients with private medical insurance. Uninsured UK residents can access this treatment through our Expanded Access Pathway
Please note, on occasions some insurers may ask for a medical report.
Refer a patient
Making a referral is easy. Please complete our online referral form or request a call back.
Benefits for patients
Avoids invasive treatment
MR-guided SABR boost offers an alternative non-invasive treatment option for patients with cervical cancer who are unable to receive brachytherapy.
Precision targeting
MR-guided SABR boost delivers a higher overall dose than IMRT or VMAT – reaching expected doses of 30-35 Gy in just 5 fractions
Protection of organs at risk (OAR)
Smaller margins defined by MRI guidance reduces exposure to surrounding healthy tissue and organs. Live tracking and automated beam gating means radiotherapy is paused if the tumour moves outside the treatment boundaries, leading to fewer side effects and improved outcomes.
Convenient treatment schedule
Treatment can be delivered in five sessions over five alternative days meaning less intrusion into the patient’s day-to-day life.

What are the inclusion criteria?
Patient eligibility
- Locally advanced (FIGO stage 1B3-IVA) or medically inoperable (FIGO stage 1) cervical cancer
- Patient is undergoing radical chemoradiotherapy or radical radiotherapy and unable to receive a brachytherapy boost
- Reviewed through NHS gynae MDT (outcome ‘radiotherapy’ is acceptable)
- Patient is aware that HDR brachytherapy is the gold-standard of treatment and that MRIdian MRI-guided SABR boost is being offered as a suitable alternative as HDR brachytherapy is not possible
- Patient fit for out-patient treatment and able to travel daily to the department for treatment
- Clinical Target Volume (CTV) <150cc (typical range 30-100cc)
- Larger CTV can be considered but should be reviewed by the clinician and planning team as early as possible
- Referral should be prior to completion of the initial course of radiotherapy. However, understandably cases may be referred in week 6 after a failed brachytherapy attempt. For these referrals it may not be possible to achieve the full treatment course within the 56-day target. The referring clinician will be updated and appropriate course of action discussed
What are the exclusion criteria?
- Patient has pacemaker, ICEDs defibrillator or neuro-stimulator
- Intra-orbital foreign bodies and metallic foreign bodies which may be affected by the MR Linac magnetic field
- Aneurysm clips
- Severe claustrophobia
- Lesion/cervix not visible on MRI
- Tumour involvement of bladder or bowel at week 5 MRI is not an absolute contra-indication but clear counselling of the patient is necessary in this circumstance
Evidence base
As chemoradiotherapy followed by brachytherapy boost remains standard of care for locally advanced cervical cancer, studies of alternatives to brachytherapy are limited. However, studies have shown external radiotherapy to be a reasonable option when brachytherapy is not an option, with more recent research supporting the use of SABR as an alternative to brachytherapy.
MR-guided SABR as an alternative to brachytherapy in cervical cancer is a cutting-edge treatment option with limited published data available in the literature. The rationale for using MRI guidance to deliver SABR is clear though and has been demonstrated in other areas of oncology. For example, the Phase III MIRAGE trial showed an advantage to patients with prostate cancer treated with MR-guided SABR compared with those treated with CT-guided SABR. MR-guided SABR is increasingly being used to treat cancers elsewhere, including in the breast, abdomen and lung.
Evidence for dose recommendation as a boost for cervix cancer is supported by the EMBRACE-1 study, where patients received external beam radiotherapy, concurrent chemotherapy and MRI-based image-guided adaptive brachytherapy (MR-IGABT). Analysis of data from 1,318 patients supported the recommendation of a dose of 85Gy to 90% of the high-risk clinical target volume (CTV) which led to a 3-year local control of 95% for squamous cell and 86% for adeno/adenosquamous carcinoma histology.
Patient selection is an important consideration. In general, MR-guided SABR boost as an alternative to brachytherapy should only be considered for patients with locally advanced cervical cancer who are unable to have brachytherapy boost. Studies using linac based SABR with high dose per fraction have raised toxicity concerns as well as lower-than-expected efficacy outcomes.
A series of trial plans have been performed at GenesisCare using a 5# regimen using the MRIdian MR-Linac and have been able to achieve mean dose of90% of the CTV of 92.3Gy, with a PTV D98 of 69.5Gy and PTVD90 of 78.7Gy whilst remaining within EMBRACE2 guidelines for organ at risk doses.
Our MR Linac specialists
At GenesisCare, MR-guided SABR is delivered by our expert team of clinical oncologists, therapy radiographers, dosimetrists and physicists, supporting by our experienced nursing team.
All referrals are reviewed by the GenesisCare SABR Advisory Team (SAT), an expert multi-disciplinary team of doctors specialising in the relevant tumour areas.
Since introducing the MRIdian MR Linac in 2019, we have provided MR-guided SABR to more than 2000 patients across a range of cancers. Together with our academic collaboration with the University of Oxford, we continue to support research and contribute to the growing body of evidence for this innovative method of treatment.

References
- Sagae, S., Toita, T., Matsuura, M., Saito, M., Matsuda, T., Sato, N., Shimizu, A., Endo, T., Fujii, M., Gaffney, D. K., & Small, W., Jr (2023). Improvement in radiation techniques for locally advanced cervical cancer during the last two decades. International journal of gynecological cancer: official journal of the International Gynecological Cancer Society, 33(8), 1295–1303. https://doi.org/10.1136/ijgc-2022-004230
- Barraclough, L. H., Swindell, R., Livsey, J. E., Hunter, R. D. & Davidson, S. E. External Beam Boost for Cancer of the Cervix Uteri When Intracavitary Therapy Cannot Be Performed. Int J Radiat Oncol Biol Phys 71, 772–778 (2008).
- Haas, J. A. et al. CyberKnife boost for patients with cervical cancer unable to undergo brachytherapy. Front Oncol 2, (2012).
- Hsieh, C. H. et al. Stereotactic body radiation therapy via helical tomotherapy to replace brachytherapy for brachytherapy-unsuitable cervical cancer patients - a preliminary result. Onco Targets Ther 6, 59–66 (2013).
- Hadi, I. et al. MR-guided SBRT boost for patients with locally advanced or recurrent gynecological cancers ineligible for brachytherapy: feasibility and early clinical experience. Radiation Oncology 17, (2022).
- Kishan, A. U. et al. Magnetic Resonance Imaging–Guided vs Computed Tomography–Guided Stereotactic Body Radiotherapy for Prostate Cancer. JAMA Oncol (2023) doi:10.1001/jamaoncol.2022.6558.
- Henke, L. E. et al. Magnetic Resonance Image-Guided Radiotherapy (MRIgRT): A 4.5-Year Clinical Experience. Clin Oncol 30, 720–727 (2018).
- Chuong, M. D. et al. Patterns of utilization and clinical adoption of 0.35 Tesla MR-guided radiation therapy in the United States – Understanding the transition to adaptive, ultra-hypofractionated treatments. Clin Transl Radiat Oncol 38, 161–168 (2023).
- Liu, Y. et al. Local Therapies for Hepatocellular Carcinoma and Role of MRI-Guided Adaptive Radiation Therapy. J Clin Med 12, 3517 (2023).
- Tallet, A. et al. Is MRI-Linac helpful in SABR treatments for liver cancer? Front Oncol 13, (2023).
- Schmid, M. P. et al. Risk Factors for Local Failure Following Chemoradiation and Magnetic Resonance Image–Guided Brachytherapy in Locally Advanced Cervical Cancer: Results From the EMBRACE-I Study. Journal of Clinical Oncology 41, 1933–1942 (2023).
- Albuquerque, K. et al. A Phase II Trial of Stereotactic Ablative Radiation Therapy as a Boost for Locally Advanced Cervical Cancer. Int J Radiat Oncol Biol Phys 106, 464–471 (2020).
- Eakin et al Brachy 2023;22:461-467 Karlsson, J., Dreifaldt, A. C., Mordhorst, L. B., & Sorbe, B. (2017). Differences in outcome for cervical cancer patients treated with or without brachytherapy. Brachytherapy, 16(1), 133–140. https://doi.org/10.1016/j.brachy.2016.09.011.