Theranostics - 2 Years On
Metastatic castration-resistant prostate cancer (mCRPC) is challenging to treat and has an average survival of just over a year.
A vision realised
In June 2021, the open-label phase 3 VISION trial highlighted the importance of 177Lutetium PSMA therapy in prolonging survival in metastatic castration-resistant prostate cancer. This highly personalised cancer treatment combines diagnostics and therapy into a novel approach often referred to as Theranostics. The results of this trial have further validated the success that GenesisCare has experienced over the last two years of treating patients with 177Lutetium PSMA in the UK. GenesisCare was the UK’s first healthcare provider to make this revolutionary treatment clinically available in May 2019.
The UK Theranostics service at GenesisCare is led by Dr Yong Du, Clinical Director – Nuclear Medicine and Theranostics. He was the first in the UK to hold an Administration of Radioactive Substances Advisory Committee (ARSAC) licence for 177Lutetium PSMA therapy. Supported by a multidisciplinary team of nuclear medicine experts, including physicians, physicists, imaging professionals and senior nursing support, Dr Yong Du has treated over 100 patients at GenesisCare, more than any other healthcare provider in the UK in both public and private sectors.
Here, Dr Du explains how this novel treatment works and why it is providing so many patients with a new sense of hope
How does it work?
The median survival of patients with metastatic castration-resistant prostate cancer is just over a year and the survival chance for five years is less than one in six. Due to these survival rates, the focus of care moves to overall survival and quality of life. GenesisCare is always searching for the latest treatments and innovations to bring our patients the highest level of care and results. Theranostics was one of the most promising new therapies being studied at the time. This molecular-based therapy seeks out and destroys cancer cells using prostate-specific membrane antigen (PSMA)-targeting radioligands. Described as the therapeutic equivalent of an intelligent homing missile, it takes a highly personalised approach. It exploits the fact that prostate-specific membrane antigen (PSMA) is highly expressed in prostate cancer and mCRPC lesions. Combining the PSMA specific ligand with 177Lutetium (a ß-emitting particle), radiation can be delivered directly to these PSMA-expressing cells and the surrounding microenvironment.
The aims of this treatment are to
- Reduce the size of the tumour and stop it from growing
- Improve the symptoms caused by the tumour
- Maintain and improve quality of life
and with the additional advantage that this is achieved with minimal side effects.
These advantages culminate in the overall goal of this direct approach treatment, to improve quality of life for our patients, and prolonging progression-free survival in metastatic disease. Over the last two years, GenesisCare has seen highly favourable results in the patients we have treated. Furthermore, the phase 3 VISION trial results have validated the positive results that GenesisCare has seen.
The mechanism is fundamental to its effectiveness. It enables us to target the radiation very accurately and thus spare non-tumour critical organs from radiation. Due to its minimal toxicity, most patients can receive multiple cycles of treatment without developing significant adverse effects
- Dr. Yong Du
The evidence base
The phase 3 VISION trial included 831 patients who had progressive PSMA positive mCRPC and had received at least one novel androgen axis drug or abiraterone acetate and were previously treated with one to two taxane regimens. Those who received 177Lutetium PSMA plus best standard of care saw a 38% reduction in risk of death with a median overall survival benefit of four months. They also saw a 60% reduction in radiographic disease progression or death risk with the median radiographic progression-free survival benefit of five months compared to the best standard of care alone.
Even with the advancement in standard of care for mCRPC over the last two years, 177Lutetium PSMA still outperformed this – making these results even more impressive. Given that we are continually improving how we deliver Theranostics, this service holds great promise for the future of treatments we offer.
In one of our Theranostics case studies, we saw a patient with progressive mCRPC who had multiple left pelvic nodal metastases, measuring up to 2cm in size. Despite chemotherapy and hormonal therapy, the patient was not suitable for stereotactic ablative radiotherapy (SABR) treatment, so an alternative treatment approach was required.
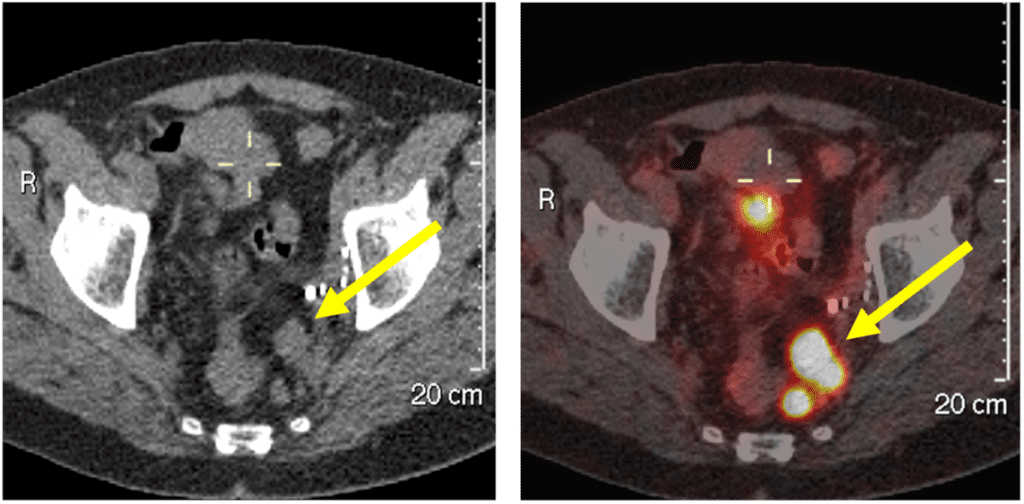
Figure 1: 68Gallium PSMA PET/CT scans before treatment
After two cycles of 177Lutetium PSMA therapy, the patient had a PSA reduction from 4.21 to 0.36 ng/ml and a reduction in tumour size from 2 cm to 0.8 cm.
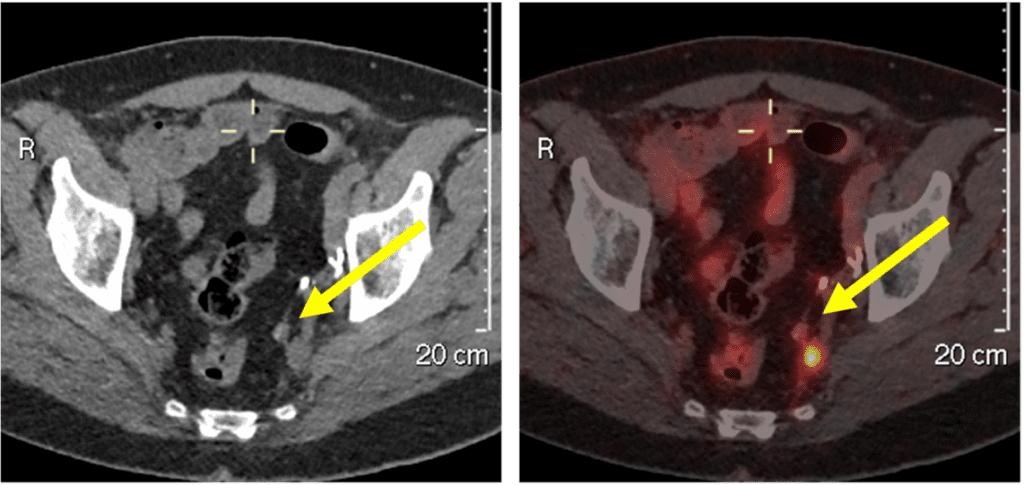
Figure 2: 68Gallium PSMA PET/CT scans after two cycles of 177Lutetium PSMA therapy
With a further three treatment cycles, the disease stabilised, and they could continue with consolidation radiotherapy. In reflection, the patient reported minimal side effects and felt more positive for the future.
This is just one case in the hundreds that GenesisCare has treated. The full detail, including 68Gallium PET/CT scans before and after treatment, can be found in our Theranostics two years on report, along with further case studies and patient testimonials.
Sharing our experience
With the many positive outcomes being seen with this treatment, further supported by the phase 3 VISION trial, the experience and knowledge that GenesisCare has gained over the last two years are invaluable. Over time, we have refined our Theranostics service and gained insights and knowledge that we readily share with colleagues around the UK in the NHS and internationally.
For example, when we started our treatment in the UK, there was limited experience of the criteria for patient selection, although our colleagues in Australia had existing expertise and protocols. Over the last two years, we have improved our ability to identify patients who will benefit the most from this treatment. Conversely, we have also learned how to identify patients who are unlikely to benefit. This comprehensive assessment and understanding of every patient’s situation mean that the right course of treatment available for metastatic disease is decided upon quickly. Producing our own 68Gallium PSMA in our on-site radiopharmacy in Windsor has also meant that patient scans can be accessed swiftly, and we can provide treatment without delay.
The unique mechanism of this treatment means that side effects are minimal. Out of more than 100 patients we have treated in the UK, treatment-related toxicity was only observed in a few patients and was usually predictable. The average dose cycle is 4 to 6, but sometimes we have had patients who received up to 10 dose cycles with no adverse effects. For each treatment, we collect patient-reported outcomes data and hope to share this information in the future.
For patients with advanced cancer, 177Lutetium PSMA therapy has offered the chance to prolong progression-free survival with an improved quality of life. Due to so many of our patients experiencing little to no side effects, they could continue with their day-to-day activities during treatment. They were also able to make the most of the extended time the treatment gave them. The feedback to this treatment has been overwhelmingly positive, and GenesisCare is proud to offer this treatment to our patients.
Innovation
Innovation has always been at the heart of GenesisCare. Every day we strive to provide our patients with the best diagnostics and treatment. Two years ago, Theranostics was not available in the UK and not widely known about or understood. Patients in the UK would have had to travel to Australia or Germany for this treatment, which would be very taxing on patients with metastatic castration-resistant prostate cancer. Following our extensive experience and evidence in GenesisCare Australia, GenesisCare invested in this treatment and brought it to the UK. Since then, we have treated over 100 patients, with many new patients currently in referral.
With the phase 3 VISION trial confirming what we have witnessed over the last two years, we now have plans to expand the availability of 177Lutetium PSMA therapy to our other centres. Alongside this, we are also in the process of developing exciting clinical trials to test innovative Theranostics procedures for other tumour types. We have led this treatment in the UK and will continue to lead.
We consider referrals for 177Lutetium PSMA therapy for patients with metastatic castration-resistant prostate cancer (mCRPC) who have exhausted or are ineligible for approved alternative options. In addition, they must demonstrate adequate uptake of PSMA ligands based on a pre-therapy imaging study to be considered for treatment. 177Lutetium PSMA therapy is delivered as an outpatient procedure at our GenesisCare centre in Windsor.
