- Healthcare Professionals
Clinics
Our clinics offer rapid access to diagnostics, with flexible appointments often within 24 hours, including breast, urology, head and neck, gynaecology, and haematology services.
Diagnostic tests and scans
Our comprehensive diagnostic service covers all modalities including pathology, radiology and genetics, led by nationally-recognised consultants and supported by dedicated on-site clinical teams.
Innovations blog
From pioneering biopsy techniques to spotting red flags of myeloma in primary care, our blog covers trending medical topics and innovative services, for healthcare professionals by healthcare professionals.
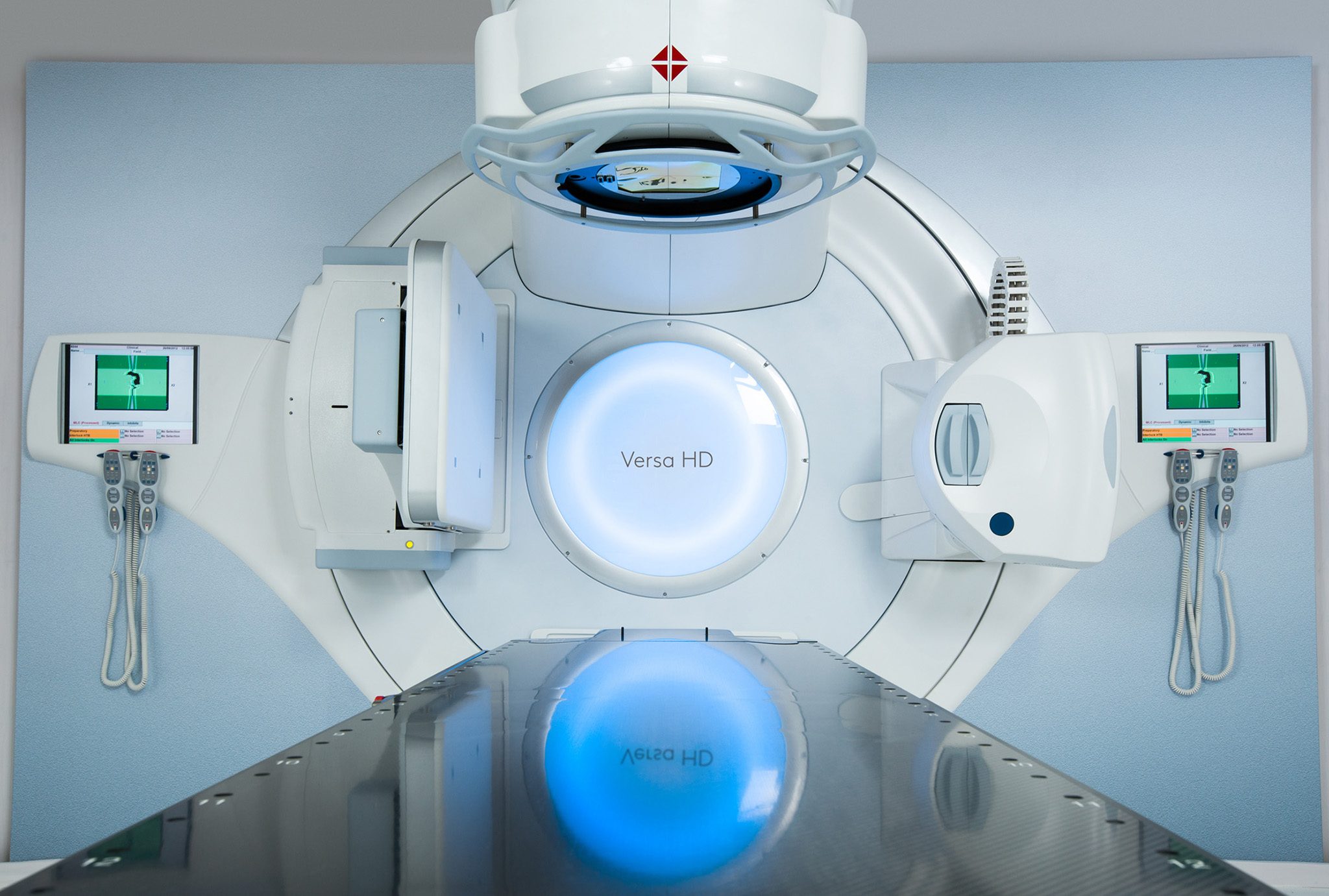
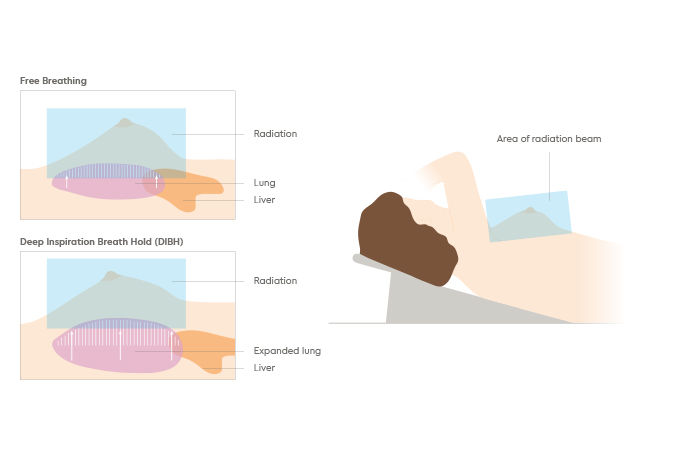
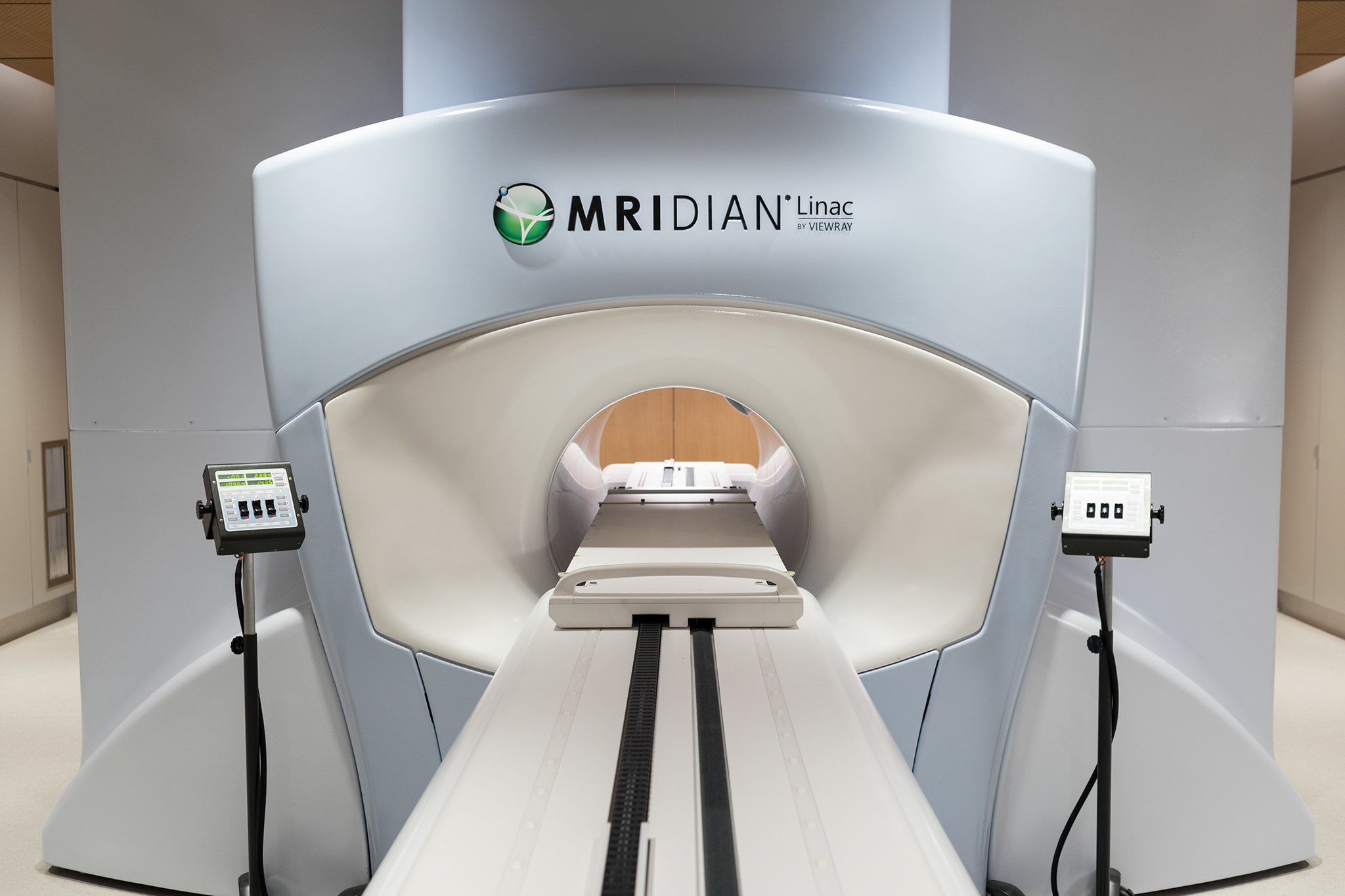
Radiotherapy
GenesisCare is at the forefront of the introduction of innovative and advanced radiotherapy techniques, such as MRIdian MRI-guided radiotherapy.
Systemic anti-cancer therapy
We provide the latest systematic anti-cancer therapies with highly trained 1-1 nursing and integrative cancer care.
Theranostics
An innovative approach using peptide receptor radionuclide therapy (PRRT) to seek out and destroy cancer cells.
Rapid access breast pathways
A world-class breast cancer pathway, with access within two weeks. GenesisCare is the UK’s leading private oncology provider, renowned for providing fast, efficient, high-quality care.
Why refer to GenesisCare?
Innovative end-to-end pathways
We invest early in the latest technologies and treatments to ensure your patients can access to world-class cancer care seamlessly from diagnostics to aftercare and follow-up.
Governance and MDTs
Through strong regulation and governance, we deliver the latest evidence-based treatments and best practice protocols in a safe and high-quality environment.
Fast and easy access
No waiting lists means your patients can access the care they need quickly at one of our 14 specialist outpatient centres throughout the UK.
Latest updates
- t1
- t2
- t4
- t5