- Patients
- Why choose GenesisCare
- Governance and safety
Governance and safety
Patient safety is our top priority. Our robust clinical governance framework ensures we’re continuously improving the quality of our services and safeguarding our high standards of care in line with evidence-based practice and professional standards
Regulatory compliance
GenesisCare is committed to providing the highest quality care to all its patients and to an environment of clinical excellence, objectivity, and accountability.
Ensuring our patients’ safety remains at the heart of what we do. We work in line with ever changing evidence-based practice and professional standards. To validate the care delivered, our centres are regularly inspected by external regulators such as the Care Quality Commission.
The Care Quality Commission (CQC)
All our centres are monitored, inspected, and regulated by the independent regulator of health and social care, the Care Quality Commission, who ensure fundamental standards of quality and safety are met. These standards are based on the expectations of our patients and those close to them.
The CQC base their inspections on five key areas:
- Safe: are you protected from abuse and avoidable harm?
- Effective: does your care, treatment and support achieve good outcomes, help you to maintain quality of life and is based on the best available evidence?
- Caring: do staff involve and treat you with compassion, kindness, dignity, and respect?
- Responsive: are services organised so that they meet your needs?
- Well-led: do the leadership, management and governance of the organisation make sure it's providing high-quality care that's based around your individual needs, that it encourages learning and innovation, and that it promotes an open and fair culture?
We’re registered with the CQC who inspect and monitor healthcare services in England and make sure they’re safe, effective, caring, responsive and well-led.
The CQC has rated 13 of our centres as outstanding or good, with the remaining centre awaiting inspection. We also review our own centres regularly and receive patient feedback scores for our high-quality care.
During 2022, 92% of our patients would recommend us to friends and family. Find out more on the CQC website.
Ionising Radiation Medical Exposure Regulations IR(ME)R
Ionising radiation is used to help diagnose and treat conditions and is used for x-rays, nuclear scans, and radiotherapy. The IR(ME)R regulations aim to ensure that it is used safely to protect individuals from the risk of harm when exposed to ionising radiation.
The CQC is responsible for enforcing the regulations.
We have robust procedures in place to protect our patients and staff from the hazards of ionising radiation. These procedures are constantly reviewed and updated. We also ensure all staff are adequately trained on radiation safety and IRMER regulations.
Safe handling of drugs used for chemotherapy
Cytotoxic drugs, which are used for chemotherapy, are hazardous substances as defined by the Control of Substances Hazardous to Health Regulations 2002 (COSHH). We have strict control measures and suitable precautions in place to protect our staff who handle these drugs. We assure ourselves of our practices and processes by adhering to evidence-based practice and professional standards and audit ourselves on the quality of our services.
Audits
GenesisCare have a comprehensive programme of clinical and quality audits which are undertaken by all our centres. This enables us to assure ourselves of the consistent and high-quality care being delivered. This includes audits on patient safety, clinical effectiveness, and management.
Incidents, Complaints, Near Misses, and Concerns
We pride ourselves on being an open and learning culture and take every opportunity to take lessons and make changes when things go wrong. We have robust governance arrangements in place to ensure all incidents are investigated and lessons are learnt. We apply the Duty of Candour professional and legal requirements where required.
Quality standards
All our centres have the Macmillan Quality Environment Mark (MQEM) which is used for assessing whether cancer care environments meet the standards required by people living with cancer.
As an organisation we hold ISO 9001 accreditation. ISO accreditation assures us of the quality of our governance processes and standards compared to an international benchmark.

Clinical leadership
Our clinical leadership structure ensures we deliver the latest evidence-based treatments in a safe and high-quality environment. This structure ensures rapid management of risks and issues but also ensures the right people are involved in quality and safety decisions at all levels.
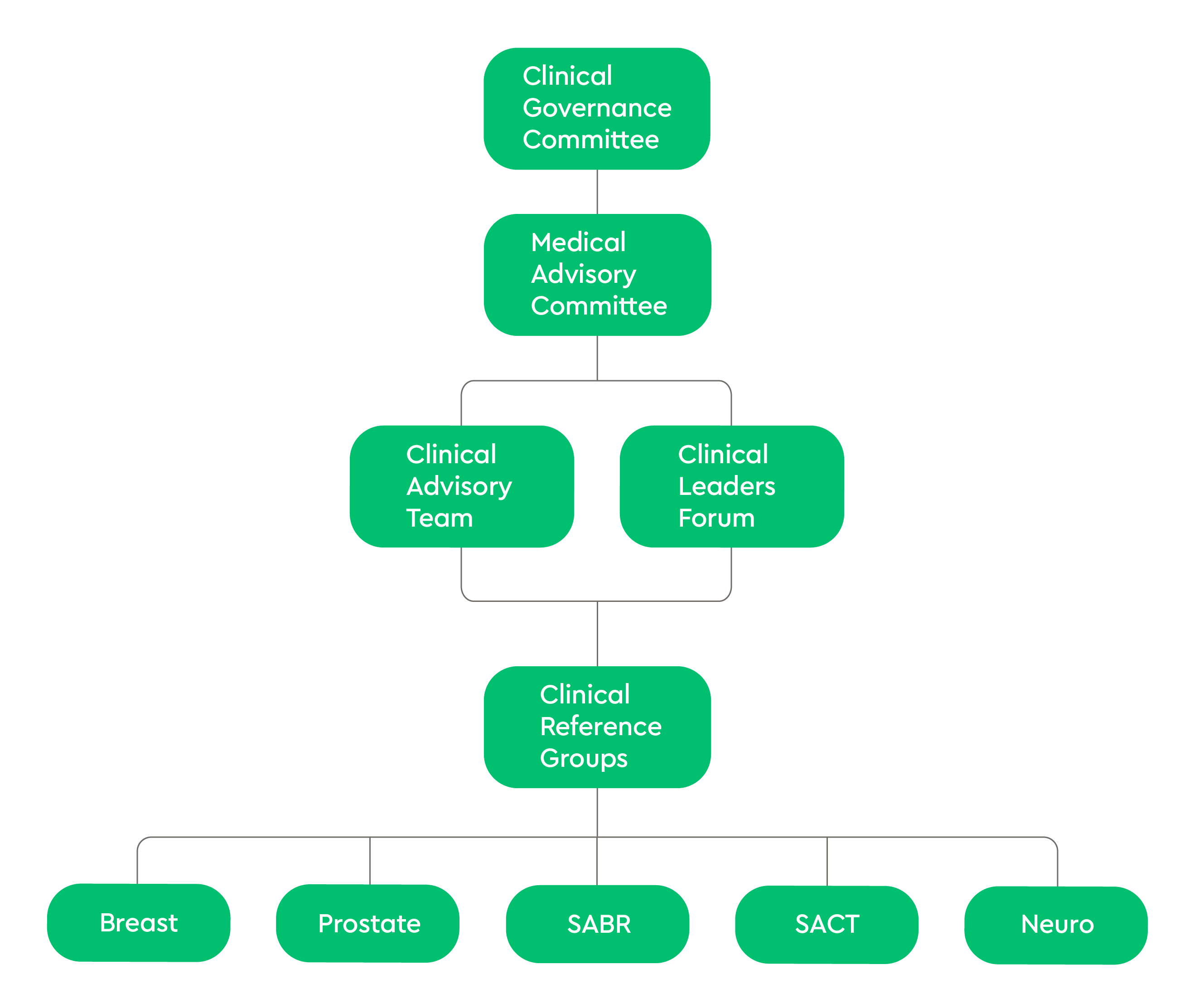
Clinical Governance Committee
The Clinical Governance Committee acts as the main quality forum within GenesisCare. At this forum, all elements of quality within the business (evidence-based practice, professional standards, learning from incidents, audit results, and emerging and managed risks) are consolidated into meaningful discussions and decision making. This forum sets the strategic direction of quality improvement within GenesisCare and acts as the epicentre of policy and process decisions. This MDT group is made up of senior leadership within GenesisCare, the quality team, clinical heads of service, and key individuals.
Medical Advisory Committee
The Medical Advisory Committee acts as the main forum for discussing individual consultants within GenesisCare. This forum of experienced oncology consultants continually reviews and validates consultants’ performance and ensures the values of GenesisCare are met.
Clinical Advisory Team
The Clinical Advisory Team reviews and recommends best treatment plans for cases outside our standard protocols
Clinical Leaders Forum
The Clinical Leaders Forum consists of leading practitioners in all fields of oncology who work with GenesisCare to spearhead best practices and innovations and expand our referral network. The consultants in this forum provide independent oversight and challenge decisions and actions of the senior leadership team, clinical heads of service and the quality team.
Clinical Reference Groups
The Clinical Reference Groups provide clinical oversight for safe and effective services across our centres and develop new techniques and treatment protocols to maintain best practice in line with both national and international developments. These groups are made up of an MDT of leading NHS and private practice clinicians, along with technical and quality experts to ensure that the patient has the most effective treatments possible, using the latest techniques in innovations in oncology.
All consultants who work with us are independent practitioners who are granted practicing privilges
Working with consultants
Most of our consultants also work in the NHS and all consultants must have practising privileges to provide treatment at our centres.
To gain practising privileges, consultants must be listed on the General Medical Council (GMC) register and fulfil a number of other criteria including demonstrating relevant clinical experience. Practising privileges are approved by our Medical Advisory Committee and are regularly reviewed.
Consultants are responsible for making clinical decisions and for the quality of care they provide. Our governance structure is responsible for ensuring consultants practise in accordance with GenesisCare policy and in line with their practising privileges.
Multidisciplinary team meetings (MDTs)
Multidisciplinary team meetings (MDTs) are clinical forums where consultants, specialist doctors, nurses and other relevant healthcare professionals come together in a meeting setting to share their expert clinical knowledge and make collective evidence-based recommendations on each individual case. The MDT meeting enables the team to discuss individual treatment plans and follow-up arrangements to ensure that every patient with cancer receives the best possible care.
All patients with a new diagnosis of cancer are discussed at an MDT meeting either from the NHS provider or through a GenesisCare MDT meeting.
Patient recall procedures
Patient recalls are very rare. But we have a number of procedures in place to ensure there is a smooth process should the need arise.
Patient are recalled if we have concerns that any treatment or behaviour of any medical professional isn’t of the correct standard. It’s our duty to make you aware of these concerns if you may have been affected.
As part of your recall, you may be provided with an independent review of your treatment. The review will be carried out by an independent specialist not involved in your original care.
Following your review, we’ll ensure you have advice, support, and follow-up, including further treatment and counselling if any of these are necessary.
Read next

Conditions
Exploring cancer care
We are the UK’s leading private provider of advanced radiotherapy and cancer care.

Treatment
Radiotherapy treatment
Radiotherapy, also called radiation therapy, kills cancer cells. It’s used in the early stages of cancer treatment or after it has started to spread.

News
Integrative cancer care
We offer therapies that are proven to help people with cancer and aim to support you through your treatment and beyond.

Research
Patient resources
We believe care should be focused on you, the individual, not your condition.

News
News room
From new centers and technologies to the latest innovations in care, stay on top of what we are doing